| Issue |
Knowl. Manag. Aquat. Ecosyst.
Number 425, 2024
Riparian ecology and management
|
|
|---|---|---|
| Article Number | 18 | |
| Number of page(s) | 15 | |
| DOI | https://doi.org/10.1051/kmae/2024015 | |
| Published online | 09 October 2024 | |
Research Paper
Habitat-based variation in the trophic ecology of the world’s highest-altitude self-sustaining population of invasive rainbow trout
1
Doctorado en Ciencias Aplicadas mención Sistemas Acuáticos, Facultad de Ciencias del Mar y Recursos Biológicos, Universidad de Antofagasta, Antofagasta, Chile
2
Fish and Stable Isotope Ecology Laboratory, Instituto de Ciencias Naturales Alexander von Humboldt, Universidad de Antofagasta, Antofagasta, Chile
3
Millenium Nucleus of Austral Invasive Salmonids (INVASAL), Concepción, Chile
4
Universidad de Antofagasta Stable Isotope Facility (UASIF), Instituto Antofagasta, Universidad de Antofagasta, Antofagasta, Chile
5
Genomics in Ecology, Evolution and Conservation Laboratory, Departamento de Zoología, Facultad de Ciencias Naturales y Oceanográficas, Casilla 160-C, Universidad de Concepción, Concepción, Chile
6
Scottish Centre for Ecology and the Natural Environment, University of Glasgow, Glasgow, UK
* Corresponding author: karina.gonzalez.flores@ua.cl; karinabe.gf@gmail.com
Received:
9
May
2024
Accepted:
27
August
2024
Rainbow trout (Oncorhynchus mykiss) have been widely introduced outside of their natural range and are one of the World’s most invasive species. Illegally introduced into the isolated, high-altitude (4520 m) Chungará catchment of northern Chile, rainbow trout have now been present in the system for ∼30 years and to our knowledge are the World’s highest known self-sustaining population. However, nothing is known about the status and impacts of these trout in the system. We analysed stomach contents and stable isotopes (δ13C, δ15N, δ34S) from liver and muscle to study the trophic ecology of rainbow trout from lake, river mouth and riverine habitats in the Chungará catchment to understand their basic ecology and to characterise their potential trophic interactions with native taxa. Both stomach contents and stable isotope values showed spatial differences in diet and habitat use, but also revealed that diet varied temporally within habitats. The trophic position of rainbow trout was similar in the three habitats examined and was constant over time, apart from the riverine population. Predation by rainbow trout had different potential effects across pelagic, benthic, and riverine habitats in the Chungará catchment, through the consumption of zooplankton, native amphipods, aquatic insects, and the endemic cypriniodontid fish Orestias chungarensis.
Key words: High–altitude freshwater ecosystems / invasive species / stable isotopes / stomach contents analysis / trophic position
© K. González et al., Published by EDP Sciences 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
1 Introduction
The introduction of non–native fish species into freshwater ecosystems is a major threat to the preservation of global biodiversity and the conservation of these ecosystems (Copp et al., 2005). Tropical high Andean lakes (> 4000 m asl) can be particularly permeable and open to biological invasions as their food webs are relatively simple compared to systems at lower altitudes e.g., Lake Titicaca (Jacobsen and Dangles, 2017). High Andean lakes have low species diversity, support few but often endemic fish species, or are devoid of native fish and have simplified planktonic food webs dominated by small algae, bacteria, and flagellates (Jacobsen and Dangles, 2017). Although tropical high Andean lakes are recognised as having high conservation biodiversity value (Myers et al., 2000), few studies have examined the ecological effects of introduced fish in these systems (Aguilera et al., 2006; Mouillet et al., 2018).
Salmonid fishes have been widely introduced into freshwater systems outside of their natural range in the Northern Hemisphere. The rainbow trout (Oncorhynchus mykiss (Walbaum, 1792)) has a native distribution that extends from Alaska to Mexico along the Pacific coast of Western North America and the Kamchatka Peninsula and Shantar Islands on the Northwestern Pacific coast of Russia (Behnke, 1992; Markevich and Esin, 2019). Over the past 150 years, rainbow trout have been introduced to rivers and lakes across the world (Crawford and Muir, 2008) where its impacts have led to the species being considered among the 100 most problematic introduced species worldwide (Lowe et al., 2000).
Salmonids are opportunistic, visual, and highly size-selective predators (Carlisle and Hawkins, 1998; Tiberti et al., 2014; MacLennan and Vinebrooke, 2016). Rainbow trout are characteristically opportunistic predators that consume a wide range of animal prey (Artigas et al., 1984; Gibson, 1988; Ferriz, 1994), allowing them to switch their diet to reflect prey availability. Where introduced, rainbow trout have been repeatedly associated with direct negative effects on receiving ecosystems and the native taxa they support (Baxter et al., 2007; Monroy et al., 2014; Mouillet et al., 2018). These effects typically reflect predation impacts on native organisms across a range of taxa, including zooplankton, macroinvertebrates, amphibians, and fish (Matthews and Knapp, 1999; Knapp et al., 2001; Martín-Torrijos et al., 2016). Although often considered as a cold-water pelagic piscivore, rainbow trout can also consume substantial amounts of zooplankton (Hubert et al., 1994; Aguilera et al., 2006) and benthic invertebrates (Vimos et al., 2015), if pelagic fish prey are not present.
Rainbow trout were first introduced to South America in 1900 (Wilcomme, 1988) and soon became a popular source of both food and sport (Macdonald and Ruxton, 2017). Given the informal manner of many introductions, information on the source, type or timing of introductions is typically absent. For instance, rainbow trout were illegally introduced into the high-altitude (4520 m) Chungará catchment in northern Chile at some point during the late 20th century, with most evidence pointing towards an introduction date in the 1990s. Prior to this, the lake and its principal tributary, the River Chungará, supported a single endemic fish, Orestias chungarensis, with the small catchment forming the species entire global distribution (Vila and Pinto, 1986; Vila et al., 2007). Currently, juvenile trout inhabit the lake’s main tributary, the River Chungará, while adults are found in the more productive lake, reaching sizes > 6.5 kg (pers. obs. authors).
To our understanding, the Chungará catchment stock is the world’s highest known self-sustaining population of rainbow trout. Although rainbow trout has been present in the catchment for more than 30 years, and its potential predation impacts have been discussed (Vila et al., 2007; Pardo and Vila, 2008; Lobos et al., 2020), almost nothing is known about its ecology in this system and its impacts on native taxa. This largely reflects the difficulties and costs of sampling this remote ecosystem and the often extreme climatic and physical conditions found in the Chungará catchment. Until recently, sampling was further complicated due to the very strict restrictions placed on the use of petrol-powered boats by conservation managers responsible for the lake. Boat access was finally permitted to the authors’ research team in 2017 after suitable funding was found for a boat powered by natural gas.
Different approaches are used to aid the understanding of fish trophic ecology, including traditional stomach content analysis and biochemical approaches (Nielsen et al., 2017; Majdi et al., 2018; Pethybridge et al., 2018; Harrod and Stallings, 2022). Dietary estimates have long been produced using stomach contents analysis (SCA): this approach provides a high taxonomic resolution, but cannot provide information from fish with empty stomachs, and only provides a snapshot that reflects consumption in the hours prior to capture (Nielsen et al., 2017; Majdi et al., 2018; Harrod and Stallings, 2022) meaning that populations of interest need to be repeatedly sampled over time. An alternative approach is to use biochemical measures such as analysis of stable isotopes (West et al., 2006; Nielsen et al., 2017; Harrod and Stallings, 2022). Stable isotope analysis provides information on prey assimilation (unlike SCA which reflects consumption) over longer temporal scales than SCA (weeks − months), with the period depending on the tissue sampled (Thomas and Crowther, 2015). The predictive enrichment of the heavy nitrogen isotope (15N) between trophic levels (i.e., 3 – 4 ‰) enables the determination of consumer trophic position (Post, 2002). The stable isotopes of carbon (δ13C) and sulfur (δ34S) typically vary between different primary production pathways (e.g. pelagic, benthic, terrestrial) and compared to nitrogen, show limited shifts between diet and consumer (McCutchan et al., 2003), which allows researchers to determine the relative contribution of different energetic pathways and use of different foraging habitats by fish (Fry, 2002; Harrod et al., 2010). The study aimed to provide a first characterisation of the trophic ecology of rainbow trout in the Chungará catchment as a means of understanding the basic ecology of this relatively newly established population. Rainbow trout in populations across their native (Arostegui and Quinn, 2018) and introduced ranges (Closs, 2024) can display intrapopulation variation in the use of habitat and resources. Here we examined whether the rainbow trout population of the Chungará catchment is spatially segregated, where different groups (e.g. life stages) rely on distinct habitats or whether individuals use habitats across the whole catchment. This information is needed to identify likely impacts of the population on native taxa and ecosystem function, especially regarding the long-term conservation of the endemic fish O. chungarensis. Although of elevated conservation concern, little is known regarding Orestias ecology (Guerrero et al., 2015). To date, this species has been largely associated with the macrophyte-dominated littoral zone (Vila and Pinto, 1986), although no sampling of deeper or pelagic waters has been carried out. Given that in other systems (e.g. Titicaca) Orestias have diversified, and multiple forms/species exist (Dejoux and iltis, 1992), it is possible that there is unrecognized trophic diversity in the Lake Chungará population.
Given the difficulties of accessing and sampling the lake, we combined different approaches to maximise the information from each fish captured, including sampling in different locations, and making comparisons over different temporal scales. As such, stomach content analysis was used to characterise diet in the short term and stable isotope ratios (δ13C, δ15N and δ34S) to examine indirect information of diet assimilation in tissues over successively longer periods, i.e., liver and muscle. These data were then used to characterise consumption patterns including spatial and temporal variation of trout from riverine, river mouth and lake habitats.
2 Material and methods
2.1 Study area
Lake Chungará (69.16° W, 18.25° S) is one of the world’s highest large lakes (4520 m asl). The Chungará catchment is located in the altiplano of Northern Chile (Fig. 1). Lake Chungará is a relatively young lake and sits adjacent to two volcanoes (Parinacota, 6380 m asl and Pomerape, 6282 m asl). It was formed due to tectonic-volcanic activity ca. 10 000 ybp (Sáez et al., 2007). Lake Chungará has a surface area of 22.5 km2 (Mühlhauser et al., 1995). It has a maximum depth of ∼35 m and is polymictic. Reflecting its high altitude, Lake Chungará is relatively cold for a tropical lake, reflecting a mean annual air temperature of 4.2 °C (Pueyo et al., 2011) and water temperature varies both with depth and season between 4 and 11 °C (Dorador et al., 2003). The lake is moderately productive (mean chlorophyll a concentration = 3.1 μg l−1 (Dorador et al., 2003)). The lake’s main tributary is the River Chungará, a small (length < 10 km, mean width = 1.8 m, mean depth = 0.4 m), groundwater-fed (Herrera et al., 2006), lower order river that drains a volcanic catchment dominated by puna vegetation, which forms wetlands (bofedales) at the ecotone between the river and terrestrial habitats (Rundel and Palma, 2000).
The Chungará catchment (283 km2) has an extremely low human population density, reflecting its remote nature. It is part of the Lauca National Park, which has been a UNESCO Biosphere Reserve since 1981 (https://en.unesco.org/biosphere/lac/lauca). Land use is restricted to grazing by domestic and wild camelids.
Until the late 20th century, the catchment supported an extremely depauperate fish community consisting of just two species, both of which are small bodied (< 15 cm) and endemic to the catchment. The cyprinodontoid Orestias chungarensis was originally reported from the lake (Vila and Pinto, 1986), but was also present in large numbers along the River Chungará in 2007 and 2008 (Harrod, unpublished). A second species, the catfish Trichomycterus chungaraensis, was described from the Vertiente de Mal Paso, a short (<1.5 km), high-gradient stream draining a slope to the west of the lake (Arratia, 1983). To date, it has not been reported from either Lake or River Chungará.
The littoral zone of Lake Chungará supports a dense curtain of macrophytes (e.g., Myriophillum elatinoides, Potamogeton filifolius) at depths between <1 and 5 m. These macrophytes provide important habitats for benthic macroinvertebrates, and feeding and breeding habitats for large numbers of aquatic birds (Rundel and Palma, 2000). The macroinvertebrate community includes aquatic insects at different stages of development as well as amphipods, bivalves and gastropods. The lake’s phytoplankton community is depauperate and is dominated by diatoms during the cold season and chlorophyceans during warmer seasons (Dorador et al., 2003). The lake supports an abundant zooplankton community, whose composition is dominated by calanoid copepods (Andrew et al., 1989) but also includes cladocerans (Mühlhauser et al., 1995).
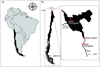 |
Fig. 1 Location of Lake Chungará in A) South America and B) Chile. The relative location of the different sampling habitats described in the text are shown. |
2.2 Sample collection
2.1.1 Fish
In November 2018 (the austral Spring), we sampled rainbow trout from their main habitats: the lake, the river, and the ecotone between these two habitats, which we will refer to as the river mouth (Fig. 1). Fish from the lake were sampled using pelagic gillnets (12.7 cm mesh × 80 m × 6 m depth) as well as using standard CEN (European Standard, 2005) benthic multipanel nets (2.5 × 1.5 m panels of 29, 35, 5, 15.5, 24, 12.5, 8, 55, 10, 6.25, 19.5 and 43 mm mesh). Nets were set in the late afternoon, fished overnight, and lifted/checked in the following morning. Rainbow trout were sampled from both the river and the river mouth (Fig. 1) by backpack depletion electrofishing (Halltech HT2000, Guelph, ON, Canada: 300 V, 60 Hz). When captured, fish were euthanised by spinal cord dislocation (approved by bioethics licence) and placed on ice before being transported to the laboratory.
2.2.2 Putative prey
No previous information was available regarding the diet of rainbow trout in the lake. As such, at the same time as collecting fish, we sampled a range of putative rainbow trout prey from the lake. Zooplankton were collected using vertical hauls of 200 μm nets (opening area: 0.125 m2, length: 100 cm, diameter: 40 cm, 6.3 cm (lower)), and filtered samples were placed in 500 mL bottles and preserved on ice. Littoral and sub-littoral macroinvertebrates were collected using a van Veen grab (volume: 400 cm2, dimensions: 550 × 340 × 220 mm) from depths of 2, 5, 10 and 15 m. Organisms were removed from the sediments, placed in labelled plastics bags, and placed on ice. 49 individuals of the endemic fish Orestias chungarensis (captured given their importance as the principal potential prey of rainbow trout) were collected as by-catch from the CEN multi-panel gillnets and were then euthanised by spinal cord dislocation. Putative prey for rainbow trout from the river mouth sampling area were obtained indirectly from rainbow trout stomach contents using a Russian doll approach (Grey et al., 2002). Putative prey from the river were collected using 0.5 mm mesh kick nets, then placed into labelled bags and stored on ice.
2.3 Laboratory work
Rainbow trout were measured for length (fork length: ±1 mm) and mass (blotted wet mass ± 0.1 g). Their stomachs were dissected out and stored in 70% ethanol. Samples of liver and white dorsal muscle were taken from each rainbow trout (lake: n = 37; river mouth: n = 18; river: n = 91), placed into 1.5 ml microcentrifuge vials, and then frozen at −20 °C. Zooplankton samples were concentrated by filtering them on GF/F filter pre-combusted (500 °C for 4 h) filters: the filter then was preserved at −20 °C.
Macroinvertebrate samples from van Veen grab were washed with distilled water and preserved at −20 °C. These were subsequently defrosted and then identified to the lowest taxonomic level using a dissection microscope and taxonomic keys (Dejoux and Iltis, 1992; González, 2003; Dominguez and Fernández, 2009) before preparation for stable isotope analyses.
Permissions:
All sampling and sample preparation was conducted under permission from the relevant agencies (SUBPESCA: R.Ex.No. 348, February 2016) and covered under the approval of the Universidad de Concepción ethic, bioethics, and biosecurity committee (CEBB permit No. 1337).
2.4 Analytical procedures
2.4.1 Stomach contents
Rainbow trout stomachs were opened, and their contents placed into Petri dishes. Prey taxa were then identified to the lowest taxonomic level using a dissection microscope using identification keys (see above). We estimated the relative importance of different prey taxa to the diet of each rainbow trout using two common approaches (Harrod and Stallings, 2022). Frequency of occurrence (%Fi) provides information on the proportion of a given taxon individuals within stomach contents and can be used as a qualitative indicator of taxa importance in the diet. The relative contribution (%Pi) provides information on the proportional contribution of food items to the stomach volume and is calculated by applying a semi-quantitative modified points method (Swynnerton and Worthington, 1940; Hyslop, 1980).
2.4.2 Determination of stable isotope ratios
Rainbow trout muscle and liver tissues, as well as putative prey samples, were dehydrated for 48 h in a benchtop freeze dryer (LABCONCO FreeZone Plus Cascade, Kansas City, MO, USA). Once dry, samples were stored in 50 mL centrifuge tubes contained in sealed plastic bags under low humidity conditions. Samples were then homogenised (Pérez et al., 2022) in 2 mL screw cap polypropylene tubes (Biologix, Shandong, China) with two 3.2 mm stainless steel beads for 30 seconds at 3500 oscillations/min using a bead-beater (Mini-Beadbeater, Bio Spec Products Inc., Bartlesville, OK, USA). After grinding, ca. 1.5 mg of homogenised tissue was weighed into 8 × 5 mm pressed standard weight tin capsules using a high precision (repeatability = 0.0008 mg) microbalance (model XS 3DU, Mettler Toledo, Greifensee, Switzerland).
Elemental percentages for carbon, nitrogen, sulfur, and stable isotope ratios (δ13C, δ15N and δ34S) were measured using a Pyrocube elemental analyser (Elementar, Langenselbold, Germany) linked to a visION continuous-flow isotope ratio mass spectrometer (Elementar, Langenselbold, Germany) at the Universidad de Antofagasta Stable Isotope Facility (UASIF), Chile. Stable isotope ratios are expressed using δ notation and are reported as per mil (‰) relative to Vienna Pee Dee Belemnite for carbon, air for nitrogen and Vienna Canyon Diablo Troilite for sulfur.
Several international standards were used in each batch to provide a multi-point calibration using the ionOS software package v4.1.005 (Elementar, Langenselbold, Germany). Certified reference materials USGS40 and USGS41a were used for carbon and nitrogen and IAEA-SO-5, IAEA-SO-6 and IAEA-S2 for sulfur. Repeated analysis of standards showed analytical errors (± 1 SD) of ± 0.04‰ for δ13C, ± 0.06‰ for δ15N and ± 0.60‰ for δ34S. We used two calibration standards, a) sulfonamide (Elementar, Germany) and b) an in-house standard (rainbow trout dorsal muscle) to correct for instrument drift.
Fish, muscle and liver tissues commonly contain lipids that interfere with the estimation of δ13C values and may have a non-dietary origin (DeNiro and Epstein, 1977). As such, we estimated lipid-free δ13C values for rainbow trout using arithmetical corrections based on sample C:N for muscle (Kiljunen et al., 2006) and liver (Logan et al., 2008). We did not correct putative prey δ13C based on the assumption that rainbow trout likely assimilate the entire biochemical package provided by prey (lipids, proteins, and carbohydrates).
2.5 Data analysis
2.5.1 Fish size
To examine possible variation in rainbow trout size in the three sampling habitats, we compared fish sizes (fork length) using the non-parametric Kruskal Wallis test.
2.5.2 Diet: stomach contents
To examine potential variation in rainbow trout diet between fish captured in the river, river mouth and lake, we calculated mean %Fi and %Pi based on 21 different dietary categories. We then arcsine square root transformed the %Pi data from each individual, and generated a Bray-Curtis dissimilarity matrix in the vegan package (Oksanen et al., 2022) for R (version 4.3.2; R Core Team, 2023) using the R Studio IDE (version 2023.10.31; RStudio Team, 2023). A non-metric multidimensional scaling (NMDS) ordination based on the Bray-Curtis dissimilarity matrix was generated to provide a visual representation of variation in rainbow trout diet composition at the individual level, as well as at the level of the three habitats sampled (lake, river mouth and river). We used the vegan function adonis2 to conduct a permutation (n = 9 999) based multiple analysis of variance (PERMANOVA) to provide a statistical comparison of rainbow trout diet between the three habitats (Anderson, 2001).
2.5.3 Stable isotope values
Stable isotope values were visualised using δ15N − δ13C, δ15N − δ34S and δ34S − δ13C biplots comparing rainbow trout and putative prey values from the lake, river mouth and river. We compared the location of the δ15N − δ13C and δ13C − δ34S centroids for liver and muscle tissues of trout captured from the different habitats using a 2-way PERMANOVA (main effects: tissue and habitat) based on a Euclidean distance matrix of untransformed δ13C, δ15N and δ34S values. To examine evidence for population-level temporal switches in δ13C, δ15N or δ34S values we compared the difference between individual liver and muscle values for each fish using one-sample t-tests for the lake, river mouth and river, and ordinary least squares linear regression. We examined possible ontogenetic diet shifts through Pearson’s correlation of fish size (fork length) and stable isotope values (δ13C, δ15N and δ34S).
2.5.4 Isotopic niche volume
We used the R package nicheROVER (Swanson et al., 2015) to calculate three-dimensional isotopic niche volume for rainbow trout captured in the three habitats. By comparing liver and muscle δ13C, δ15N and δ34S values, we were able to estimate niche size and pairwise niche overlap between habitats, and also examine evidence for temporal shifts. The isotopic niche was defined as a volume with a 95 % probability of finding a specific sample from the group (tissue/habitat) of interest and was denoted as NR95. Posterior distributions were obtained for each group (10 000 runs), and the niche size and overlap estimated. Levels of overlap were assessed by comparing posterior means of niche overlap with 95 % credible intervals.
2.5.5 Stable isotope mixing models: isotopic source proportions
Orestias chungarensis unexpectedly showed considerable isotopic variation with individuals falling into two broad classes. One group (which we refer to hence forward as Orestias A) were relatively enriched in 15N and 34S and depleted in 13C, while others (Orestias B) relatively depleted in 15N and 34S, and enriched in 13C. As such, we split Orestias into two groups whose contributions were estimated separately: δ15N (Orestias A δ15N > 11.1 ‰ − Orestias B δ15N ≤ 11.1 ‰), δ13C (Orestias A δ13C < −12 ‰ − Orestias B δ13C > −12 ‰) and δ34S (Orestias A δ34S > −4.1 ‰ − Orestias B δ34S < −4.1 ‰).
We estimated the relative contribution of different putative prey to the assimilated diet of rainbow trout using the Bayesian mixing model simmr in R (Parnell, 2016). We used mean ± SD trophic discrimination factors for C and N for liver and muscle from Canseco et al., (2021) : δ13Cliver (0.6 ± 1.3 ‰), δ15Nliver (2.8 ± 1.6 ‰), δ13Cmuscle (1.7 ± 2.0 ‰) and δ15Nmuscle (3.7 ± 3.2 ‰). For S, we used mean ±SD values from an unpublished meta-analysis (Harrod, unpublished): δ34Sliver (1.2 ± 0.1 ‰) and δ34Smuscle (1.3 ± 1.3 ‰). Models were originally run using eight distinct sources (Syrphidae, Dytiscidae, Corixidae, Zooplankton, Amphipods, Orestias A and Orestias B) reflecting their contribution to stomach contents (Tab. S1). We then combined results a-posteriori into broad functional groups that differed by sample habitat.
2.5.6 Trophic position
We calculated trophic position (TP) for the river, river mouth and lake rainbow trout populations using stomach content values, and muscle and liver tissue stable isotope values.
To calculate individual level TP using stomach contents we assigned a general estimated trophic position to prey according to their feeding habits. Zooplankton and gastropods (Biomphalaria sp.) were assigned a TP of 2. Amphipoda (Hyallela sp.), Chironomidae (larvae and pupae), Coleoptera (Dytiscidae), Diptera larvae (Syrphidae), Hemiptera and ostracods, were assigned a TP value of 2.5. Orestias chungarensis were assigned a TP of 3.2 reflecting their stomach contents (in prep. González et al.). TP was calculated for each rainbow trout individual using the equation: TPSCAi = 1+∑j=1n TPj (Pij), where Pij is the proportion of prey item j in the diet of species i and TPj is the trophic position of prey item j (Adams et al., 1983; Winemiller, 1990). To examine possible variation in rainbow trout TP in the three sampling habitats, we compared TP using the non-parametric Kruskal Wallis test.
We estimated population-level TP from stable isotope values using the Bayesian R package tRophicPosition (Quezada-Romegialli et al., 2018) using the Onebaseline model. We used amphipod δ15N as the baseline, assuming a mean amphipod trophic position (λ) of 2.5. The tRophicPosition package estimates TP using the following equation: δ15Nc = δ15Nb + ΔN (TP-λ) where δ15Nc and δ15Nb refer to the δ15N values of consumers and a single baseline, respectively, ΔN is the trophic discrimination factor (TDF) for nitrogen (N), TP is the calculated trophic position of the consumer, and λ is the trophic position of the baseline (Vander Zanden et al., 1997). As in the mixing model, we used a TDF (mean ± SD) for δ15N values of 2.8 ± 1.6 ‰ for liver and 3.7 ± 3.2 ‰ for muscle following Canseco et al., (2021).
3 Results
3.1 Fish size
Rainbow trout captured from the three habitats varied in size (Fig. 2) (Kruskal − Wallis Test: χ2 = 102.4, P < 0.0001). Fish from the river varied between 61 and 210 mm (mean FL= 124 ± 35 mm), while those from the river mouth were larger, varying between 136 and 396 mm (mean FL = 260 ± 74 mm). Fish from the lake were larger (between 270 and 770 mm; mean ± SD = 606 ± 116 mm) than those from river and river mouth habitats.
 |
Fig. 2 Histogram showing the fork length (FL) in mm of rainbow trout caught in the lake (gillnet, n = 37), river mouth (electrofishing, n = 18) and river (electrofishing, n = 91). |
3.2 Stomach contents
Rainbow trout stomach contents (Fig. 3, Tab. S1) were notably different between the three habitats. For rainbow trout captured from the lake, both measures of diet (frequency of occurrence and proportional contribution of prey) were dominated by amphipods (%Fi = 100; %Pi = 90) and the endemic fish, O. chungarensis (%Fi = 24; %Pi = 7). Other prey items included different developmental stages of various aquatic insects (Coleoptera, Hemiptera, Diptera), as well as gastropods and ostracods. Conversely, rainbow trout from the river mouth largely consumed aquatic insects, benthic macroinvertebrates, and fish: Hemiptera (%Fi = 94; %Pi = 35), Chironomidae (%Fi = 52; %Pi = 27), Amphipoda (%Fi = 89; %Pi = 18) and fish (%Fi = 29; %Pi = 7). Rainbow trout from the river had consumed different developmental stages of various aquatic insects and benthic macroinvertebrates: Plecoptera (%Fi = 85; %Pi = 45), Amphipoda (%Fi = 80; %Pi = 29) and Elmidae (%Fi = 47; %Pi = 10).
A NMDS ordination (Fig. 3) showed a clear separation between trout captured in the three habitats, as well as evidence for increased inter-individual variation in the diet of rainbow trout from river mouth and river populations compared to individuals from the lake. Differences in the diet of rainbow trout from the three habitats had strong statistical support (PERMANOVA: F2,145 = 84.3; P = 0.0001).
 |
Fig. 3 NMDS ordination biplot (2d stress = 0.13) based on Bray-Curtis dissimilarity showing variation in NMDS 1 and 2 scores based on the contents of individual rainbow trout stomachs for fish sampled in three different habitats. Each point reflects a single individual, and points located closer together have stomach contents that are more similar than those further away. Significant taxa in the diet (those explaining most of the variance among fish) and their vector loadings are indicated in black. Note the trophic segregation between capture habitats (i.e. lake, river mouth, and river trout all fall into distinct ordination space) and the reduced inter-individual variation shown by lake fish in comparison to the other habitats. |
3.3 Stable isotope values
Comparisons of stable isotope values between rainbow trout and their putative prey are shown in Figure 4. Rainbow trout from the three habitats were isotopically distinct. Lake rainbow trout muscle δ13C values varied between −16.0 and −13.6 ‰ with a mean ± SD of −15.0 ± 0.7 ‰. δ15N values showed less variation, ranging between 13.1 and 14.6 ‰, with a mean of 14.0 ± 0.3 ‰. δ34S values varied considerably, with a range of −13. 6 to −1.96 ‰ and a mean of −8.0 ± 2.8 ‰. River mouth rainbow trout were 13C-enriched relative to conspecifics from the lake, with individual δ13C values ranging between −10.2 and −7.2 ‰ with a mean of −8.4 ± 0.7 ‰. Trout from the river mouth were 15N depleted compared to the ones of the lake, varying between 8.2 and 11.2 ‰ with a mean of 9.6 ± 0.7 ‰. They were also 34S-enriched relative to the lake fish, ranging in δ34S between −9.1 and −0.5 ‰ with a mean of −2.2 ± 1.2 ‰. River rainbow trout muscle δ13C values were 13C − depleted relative to conspecifics from both the river mouth and the lake and varied between −23.2 and −17.2 ‰ with a mean ± SD of −18.9 ± 0.7 ‰. They were also 15N depleted compared to the lake and river mouth fish, varying between 6.7 and 9.7 ‰ with a mean of 7.4 ± 0.4 ‰. Rainbow trout from the river were also 34S-enriched relative to the lake and river mouth fish, varying between −0.1 ± 8.57 with a mean of 2.3 ± 2.6 ‰.
We compared δ13C, δ15N and δ34S values from the muscle and liver of each rainbow trout captured in the three different habitats (Fig. 5). One sample t-tests showed that the mean difference varied significantly (P < 0.05) from zero (i.e., the two tissues had different isotope values) for all comparisons apart from δ13C and δ34S in rainbow trout from the river mouth (P = 0.1; P = 0.6). In the lake, liver tissues were relatively depleted in 13C (mean difference = 0.4 ‰) and 34S (mean difference = 2.4 ‰) compared to muscle tissues. In the river mouth, liver samples were 15N depleted (mean difference = 1.1 ‰) compared to muscle tissues. In the river, liver samples were relatively enriched in 13C (mean difference = 0.5 ‰), depleted in 15N (mean difference = 1.7 ‰) and in 34S (mean difference = 2.2 ‰) compared to muscle tissues.
Further evidence for isotopic shifts were provided by the results of linear regression (Tab. 1), which indicated the lack of robust general relationships between individual liver and muscle stable isotope values. In only three out of nine comparisons rainbow trout liver and muscle tissue isotope values were closely related (river δ13C: R2 = 0.47, river δ15N: R2 = 0.79 and river mouth δ15N: R2 = 0.58).
Two-way PERMANOVA comparisons of combined δ13C, δ15N and δ34S values showed that the main driver of variation in rainbow trout stable isotope values was habitat (F2,286 = 1185.6, R2 = 0.9, P = 0.0001), compared to tissue (F1,286 = 75.1, R2 = 0.03, P = 0.0001). There was a significant interaction between habitat and tissue (F2,286 = 9.5, R2 = 0.006, P = 0.0001), showing that the inter-tissue shifts in stable isotope values differed across habitats.
Comparisons of fish size (FL) and stable isotope values (δ13C, δ15N and δ34S) are shown in Table 2. There was evidence for a moderate positive correlation between FL and liver and muscle δ15N values in rainbow trout captured from the lake. Rainbow trout from the river mouth showed stronger positive correlations between size and δ15N in both liver and muscle tissues, and a moderate positive correlation between FL and muscle δ34S. Rainbow trout from the river showed weak correlations between FL and liver δ13C (negative), and FL and δ34S (positive).
 |
Fig. 4 Stable isotope A) δ15N − δ13C, B) δ15N − δ34S and C) δ13C − δ34S biplots showing variation in individual values for rainbow trout liver (brown filled circles), muscle (orange filled circle), and putative prey (filled triangles) from different habitats. Note that these are data uncorrected for trophic discrimination, and that δ13C values for rainbow trout (but not their prey) have been corrected for lipid effects. |
 |
Fig. 5 Box plots comparing the distribution of carbon, nitrogen, and sulphur stable isotope values from liver (dark brown) and muscle (orange) tissue from rainbow trout captured from the lake, river mouth and river of Chungará catchment. Solid horizontal lines represent the median values; boxes represent the interquartile range (IQR) and whiskers represent the variability beyond the IQR (i.e. Q1–1.5*IQR, Q3 + 1.5*IQR). T-test results reflect the significance of pairwise comparisons of each isotope values at individual level from liver and muscle tissue in each habitat (one sample t-test). |
Results of linear regression of individual liver stable isotope values (y) on muscle stable isotope values (x) for rainbow trout captured in the three different habitats sampled in Chungará catchment. Errors are ± 1 S.E.
Results of Pearson’s correlation tests examining relations between rainbow trout size (FL, mm) and stable isotope values. Results are shown separately for liver and muscle tissues and for fish captured from the lake, the river mouth, and the river in the Chungará catchment.
3.4 Isotopic niche volume
Isotopic niche volume (95 %) estimated using δ13C, δ15N and δ34S values showed that rainbow trout captured from the lake had a similar isotopic niche size to fish from the river mouth based on values estimated from liver tissues (mean ± SE liver isotopic niche volume: lake = 40.3 ± 8.1 ‰3; river mouth = 59.0 ± 16.8 ‰3). A similar comparison using muscle tissue indicated a slight decrease in the size of the isotopic niche compared to liver in both habitats (lake: 28.1 ± 5.8 ‰3; river mouth: 56.3 ± 16.0 ‰3). River rainbow trout had markedly different isotopic niches based on the two tissues examined, with liver (15.4 ± 1.9 ‰3) having a markedly smaller isotopic niche than that indicated by muscle (64.5 ± 8.4 ‰3). Rainbow trout from the river had a far smaller isotopic niche based on liver tissues compared to conspecifics from the lake and river mouth, but a larger niche when estimated from muscle tissues.
Comparisons of isotopic niche overlap between liver (reflecting short term diet) and muscle (longer-term diet) tissues sampled from the same individual differed between habitats. Within the lake, niche volumes showed an average overlap between tissues of 69.9 %, with a slightly reduced level of overlap (53 %) recorded from river mouth fish. There was an almost total lack of between-tissue isotopic niche overlap (0.4 %) for riverine rainbow trout.
Inter-habitat comparisons showed that the isotopic niche of lake, river mouth and river rainbow trout had zero overlap both over the short- (liver: 0 % overlap) and longer-term (muscle: 0 % overlap). The lack of overlap in isotopic niche from both liver and muscle tissues provides more support for clear and marked isotopic differences in rainbow trout captured from the three different habitats.
3.5 Stable isotope mixing models: isotopic source proportions
Mixing model results (Fig. 6, Tab. S2) provided more evidence that rainbow trout diet varied considerably both between habitats, and over time within a given habitat. Based on liver (a relatively short-term indicator), rainbow trout from the lake assimilated much of their diet from zooplankton (median contribution = 27 %), Orestias (combined = 46 %: Orestias A = 34 %, Orestias B = 12 %) and aquatic insects (23 %). Models using muscle tissue isotope values indicated a slight increased contribution of zooplankton (33 %) and aquatic insects (38 %) and a slight decrease in the contribution of Orestias (22 %: Orestias A = 12 %; Orestias B = 10 %). Contributions from the remaining prey groups were low (< 8 %).
Mixing models based on liver tissues indicated that river mouth rainbow trout largely consumed aquatic insects (46 %) and Orestias (22 %) over the short-term, with zooplankton (16 %) and amphipods (10 %) making slightly lower contributions. Muscle tissue-based models suggested that over the longer term, amphipods (32 %), aquatic insects (31 %), Orestias (24 %) and zooplankton (10 %) dominated the assimilated diet of rainbow trout from the river mouth.
Mixing models using liver tissues suggested that rainbow trout from the river largely consumed aquatic insects (67 %) and amphipods (33 %). Muscle tissue-based models indicated a much increased contribution by aquatic insects (93 %) relative to amphipods (7 %).
 |
Fig. 6 Visual representation of simmr mixing model results by habitat and tissue type. Boxplots showing the distribution of posterior estimates of the contribution of the different putative prey to assimilated diet to rainbow trout from the lake (A: liver, B: muscle), river mouth (C: liver, D: muscle), and river (E: liver, F: muscle). Note that the dietary categories differ between habitats. Solid horizontal lines represent the median values; boxes represent the interquartile range (IQR), and whiskers represent the variability beyond the IQR (i.e. Q1–1.5*IQR, Q3 + 1.5*IQR). |
3.6 Trophic position
3.6.1 TPSCA
Based on stomach contents, rainbow trout from the three habitats had generally similar trophic positions (Median (± IQR) TPSCA: lake = 3.5 (± 0.1); river mouth = 3.5 (± 0.1); river = 3.5 (± 0.0). Although qualitatively similar, statistical comparisons indicated that TPSCA did vary between habitats (Kruskal–Wallis χ2= 21.2, P < 0.001) with post-hoc comparisons showing that differences were driven in TP between rainbow trout from lake and river habitats (P < 0.001).
3.6.2 TPSIA
Bayesian estimates of trophic position based on liver and muscle stables isotope values indicated that rainbow trout had a consistent TPSIA across habitats and time. Although there were some differences in median TPSIA values, 95 % credibility limits largely overlapped using both liver (lake = 3.6 (3.5 – 3.8); river mouth = 3.4 (3.0 – 3.8); river = 3.1 (3.0 – 3.2)) and muscle (lake = 3.6 (3.5 – 3.8); river mouth = 3.7 (3.4 – 4.1), river = 3.6 (3.4 – 3.7) tissues. The exception to this was TPSIA values based on liver tissues from riverine rainbow trout, which were lower (i.e. 95 % credibility intervals did not overlap) than those from the lake (liver and muscle), the river mouth (muscle) and the river (muscle).
4 Discussion
Our study provides a detailed characterisation of the trophic ecology of non-native rainbow trout from three different habitats in a high-altitude (4500 m asl) catchment. To our knowledge, this is the world’s highest self-sustaining rainbow trout population. The colonisation of a lake and river system that previously only supported a single, small-bodied endemic fish has taken place only relatively recently, i.e. in the last 2-3 decades. The Chungará system supports a thriving rainbow trout population that several decades after their putative first introduction, now occupy and dominate food webs in lotic and lentic habitats across the catchment.
As seen in populations across their native (Arostegui and Quinn, 2018) and introduced ranges (Closs, 2024), there was marked evidence of intrapopulation variation in the use of habitat and resources by rainbow trout in the Chungará catchment. Our results showed a clear structuring between the three different habitats examined in terms of fish size and trophic ecology. Although there was evidence of temporal shifts in rainbow trout diet within each habitat, inter-habitat differences were maintained across different timescales.
Stomach content analysis showed that lake rainbow trout mainly fed on amphipods and the native endemic fish Orestias. Trout captured in the river mouth consumed littoral prey such as aquatic insects, as well as amphipods, and Orestias, while riverine rainbow trout largely consumed aquatic insects. Trout captured from river and river mouth habitats showed notably more inter-individual dietary variation than lake trout.
We used stable isotope analysis to assess diet over the relative short- (liver) and long-term (muscle) from individual fish. Analyses of stable isotopes and stomach contents generally agreed within habitats, with the exception of lake fish. Analysis of stomach contents showed no evidence of large-scale consumption of zooplankton by lake rainbow trout, while mixing models based on stable isotopes values indicated that a considerable component of somatic growth (∼30 %) was fuelled by zooplankton. Although we do not have quantitative estimates of zooplankton density from Lake Chungará, they are extremely abundant, with single vertical hauls resulting in zooplankton nets becoming clogged (authors pers. obs. authors). The community is dominated by calanoid copepods, but also includes large numbers of Cladocera (Andrew et al., 1989; Mühlhauser et al., 1995). Although typically considered as invertivores or piscivores, lacustrine rainbow trout can be zooplanktivorous in both their native and introduced ranges (Jiménez − Seinos et al., 2023) to the point where they can impact water quality through top-down effects (Hembre and Megard, 2005). However, rainbow trout generally prefer Cladocera over copepods (Schneidervin and Hubert, 1987; Lynott et al., 1995).
The dissimilarity in the relative importance of zooplankton associated with stomach contents and stable isotope analyses may reflect several, possibly interacting factors. Zooplankton may be more rapidly digested than other, more resilient prey (Gannon, 1976; Nannini et al., 2016) meaning that they may have been underreported from stomach contents, although in the authors’ experience, zooplankton remain obvious in stomach contents even when highly digested. Alternatively, the consumption of zooplankton by lake rainbow trout could vary seasonally and our sampling activities did not cover the whole annual period. Indeed, prey do likely show seasonal variation in their stable isotope values, and zooplankton can show considerable and rapid shifts, given their fast isotopic turnover times relative to fish (O’Reilly et al., 2002; Harrod and Grey, 2006). Future sampling of Lake Chungará should be extended to consider seasonal variation in rainbow trout stomach contents and of the stable isotopes of rainbow trout and their prey.
Although they had fed on different prey, rainbow trout from the three habitats fed at qualitatively similar trophic positions over both the short- (TPSCA = Range 3 – 3.9) and long-term term (TPSIA = Range 3.1 – 3.7). This is of interest regarding ecosystem function and the transfer of energy and nutrients through the food web, as rainbow trout fed at similar trophic position across the wider catchment, even though they show marked disparity in size between the three habitats.
Although their trophic positions were similar, rainbow trout captured in the three habitats had distinct diets, stable isotope values and isotopic niche volumes. At a whole-catchment level, the variation shown in rainbow trout isotope values from the different habitats indicate that individuals forage in spatially separated food webs with markedly different isotopic properties (e.g. with different baselines for δ15N and δ34S).
Our results showed consistent differences in the location of the isotopic niches for rainbow trout captured from the three habitats, and this was consistent over time. Although the estimated size of the isotopic niche was generally similar in river mouth and lake trout, comparisons between liver and muscle tissues provided evidence for isotopic shifts between time scales (niche overlap 53 − 64 %). Rainbow trout captured from the river showed very distinct isotopic niche volumes depending on the time scale. The long-term isotopic niche based on muscle tissue was larger than that from lake or river mouth fish, but extremely reduced when estimated from liver tissues, with almost zero overlap between the two tissues. Taken together, these results support an argument that rainbow trout can show marked trophic diversity across both spatial (whole catchment) and temporal (short- and long-term) scales.
The level of trophic diversity displayed by the recently established rainbow trout population likely results in substantial effects on native taxa across different levels of biological organisation across the Chungará catchment. How detrimental this is to native taxa and the function of the Chungará system is unknown, but the presence of rainbow trout is associated with the loss of the endemic fish O. chungarensis from the River Chungará (CH, pers. obs.).
Orestias chungarensis have not been studied in detail, with most studies relying on individuals captured from the shallow littoral. This study is the first to have benefited from access to deeper water and we revealed an unexpected level of intra-individual variation in O. chungarensis stable isotope values. Given that stable isotope values provide an individual-level record of trophic ecology and habitat use (Harrod et al., 2005, 2010), we are confident that the Orestias population is much more ecologically diverse than previously considered and the patterns shown are likely driven by individuals foraging in different habitats or on different prey. Given the remarkable plasticity found in the genus (Monroy et al., 2014), it may reflect the existence of a previously an unrecognized ecotype or may reflect a response to competition following the invasion by rainbow trout (Vander Zanden et al., 1999).
Although our work shows that the rainbow trout population in the Chungará catchment is clearly segregated into at least three trophic groups (lake, river mouth and river), we currently do not have sufficient evidence to explicitly conclude whether this represents the existence of separate fluvial and lacustrine ecotypes as sometimes seen in their native distribution (Arostegui and Quinn, 2018) or simply segregation by rainbow trout life stage as part of the lacustrine-adfluvial migration pattern typical in potamodromous rainbow trout (Northcote, 1997).
To our understanding, rainbow trout were stocked in the River Chungará from a single source (eggs provided from hatcheries in the Bolivian Altiplano). If the Chungará catchment does indeed support separate fluvial and adfluvial ecotypes of rainbow trout, this young population must have undergone extremely rapid (<50 years), contemporary adaptation to a new environment. Similar rapid (∼100 years) adaptation has been reported in rainbow trout in their native distributions (Pearse et al., 2014). The repeated patterns of ecological divergence of habitat use and diet seen in salmonids tends to occur in lakes with depauperate fish communities, resulting in the presence of relatively unoccupied niche space (Robinson and Wilson, 1994; Skúlason and Smith, 1995; Taylor, 1999; Quinn, 2018). Such conditions certainly existed in the Chungará catchment, where prior to the introduction of rainbow trout, the lake and river waters supported a single, small-bodied endemic fish, Orestias chungarensis. Subsequently, rainbow trout have successfully utilised and dominated niches that were either inhabited by Orestias (river, lake littoral) or that were likely vacant at the time of their colonisation (lake pelagic).
The potential divergence by rainbow trout into adfluvial and fluvial sub-populations in the Chungará catchment is of clear interest to both evolutionary and conservation ecologists, especially when considered from the view of the conservation of Orestias chungarensis. If there has been an ecological divergence it adds previously unrecognised complexity to the problem of Orestias conservation. Following the expansion of the rainbow trout population, Orestias are now extinct from the River Chungará, even though they were so abundant in 2007 that the senior author was able to easily capture these fish by hand. This parallels the situation elsewhere in South America where native fishes have been displaced from riverine habitats by trout (Alonso et al., 2024).
Although still abundant in Lake Chungará, Orestias potentially face the twin impacts of predation and trophic competition from rainbow trout. There is a pressing need to conserve Orestias in the system, and this should include an aim to see the endemic fish re-established in the River Chungará where it previously thrived.
5 Conclusions
Tropical high-altitude Andean lakes are recognised as having high socio-ecological value that relies on their biodiversity (Myers et al., 2000). However, these ecosystems are remarkedly understudied, with little baseline information is available regarding their ecology. Our study provides the first ecological characterization of upper trophic level consumers in the Chungará catchment. We have improved our understanding of the rainbow trout ecology, a key invasive species, in a sensitive high-altitude freshwater ecosystem. We have shown that the trophic ecology of rainbow trout not only fell into three broad trophic groups, but that these groups differed in habitat use.
This study also reveals the utility of using combined approach integrating temporal scales of isotope analysis to rapidly characterise diet in extreme, remote ecosystems and to reveal otherwise cryptic population segregation. These findings reflect the availability of resources and ecological plasticity of rainbow trout and their ability to use a wide range of habitats in this fragile ecosystem. This characteristic drives the success of invasive species in an invaded system (Smith, 1985) and may have extended to the establishment of multiple rainbow trout ecotypes in the Chungará catchment. The trophic diversity shown by rainbow trout likely amplifies their detrimental effects on native fauna, especially on the endemic fish Orestias chungarensis which is now absent in the River Chungará. Future work is necessary to examine the unexpected isotopic variation seen in O. chungarensis, which likely represents cryptic inter-individual variation in their diet and habitat use. A deeper understanding of the trophic interactions between Orestias and rainbow trout is required as it likely include trophic overlap as well as predation. Finally, a key future objective for the Chungará catchment is the need to conclusively show the presence or absence of divergent migratory ecotypes of rainbow trout in the system, given its potential to complicate the long-term conservation of the endemic Orestias chungarensis.
Supplementary Material
Table S1. Summary statistics for rainbow trout stomach contents from three habitats in Chungará catchment. Data are provided as mean percent frequency of occurrence ( %Fi, which represents the proportion of total stomachs that contained a given type of prey) and contribution ( %Pi, which represents the proportion contribution of each food item) by study area.
Table S2. Summary statistics (median (95 % credibility limits)) for the estimated percent contribution of different putative prey to the assimilated diet of rainbow trout short (liver) and long-time (muscle) in different habitats. An em-dash indicates that a particular prey type was not included in the analysis.
Access hereAcknowledgements
This study was funded by Millennium Nucleus of Austral Invasive Salmonids (INVASAL, Project NCN2021_056) funded by Chile’s government program, ANID Millennium Science Initiative of the Ministerio de Ciencias, Tecnología, Conocimiento e Innovación. K.G. was supported by Beca de Doctorado Nacional of Agencia Nacional de Investigación y Desarrollo (ANID) and Beca de Excelencia Académica of Escuela de Postgrado from the Universidad de Antofagasta.
We thank CONAF (Chile) for the facilities provided in Parque Nacional Lauca. Special thanks to the HarrodLab and INVASAL research groups, especially to Jimmy Villanueva, Mauricio Cañas, Poliana Strange, Claudio Quezada and Chris Díaz, for their assistance in collecting and processing samples. Tamara Contador and Gonzalo Salazar are thanked for their support in invertebrate identification.
References
- Adams SM, Kimmel BL, Ploskey GR. 1983. Sources of organic matter for reservoir fish production: a trophic-dynamics analysis. Can J Fish Aquat Sci 40: 1480–1495. [CrossRef] [Google Scholar]
- Aguilera X, Declerck S, De Meester L, Maldonado M, Ollevier F. 2006. Tropical high Andes lakes: A limnological survey and an assessment of exotic rainbow trout (Oncorhynchus mykiss). Limnologica 36: 258–268. [CrossRef] [Google Scholar]
- Alonso M, Rechencq M, Lallement M, Zattara E, Fernández MV, Lippolt G, Vigliano P, Macchi PJ. 2024. Ecological traits and fishery of the Upper Limay River: a key system for salmonids in the Andean North Patagonia. In Lobon-Cervia J, Budy P, Gresswell R, eds. Advances in the Ecology of Stream-Dwelling Salmonids. Cham: Springer International Publishing 655–673. [CrossRef] [Google Scholar]
- Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol 26: 32–46. [Google Scholar]
- Andrew TE, Cabrera S, Montecino V. 1989. Diurnal changes in zooplankton respiration rates and the phytoplankton activity in two Chilean lakes. Hydrobiologia 175: 121–135. [CrossRef] [Google Scholar]
- Arostegui MC, Quinn TP. 2018. Trophic ecology of nonanadromous rainbow trout in a post-glacial lake system: Partial convergence of adfluvial and fluvial forms. Can J Zool 96: 818–827. [CrossRef] [Google Scholar]
- Arratia G. 1983. Trichomycterus chungaraensis n. sp. and Trichomycterus laucaensis n. sp. (Pisces, Siluriformes, Trichomycteridae) from the High Andean Range. Stud Neotrop Fauna Environ 18: 65–87. [CrossRef] [Google Scholar]
- Artigas JN, Campusano E, González U. 1984. Contribución al conocimiento de la biología y hábitos alimentarios de Salmo gairdneri (Richardson, 1836) en lago Naja (Chile). Gayana 49: 3–29. [Google Scholar]
- Balon EK. 1975. Reproductive Guilds of Fishes: A Proposal and Definition. J Fish Res Board Can 32: 821–864. [CrossRef] [Google Scholar]
- Baxter CV, Fausch KD, Murakami M, Chapman PL. 2007. Invading rainbow trout usurp a terrestrial prey subsidy from native charr and reduce their growth and abundance. Oecologia 153: 461–470. [CrossRef] [PubMed] [Google Scholar]
- Behnke R. 1992. Native trout of Western North America. Monograph 6, American Fisheries Society, Bethesda, Maryland, 275 p. [Google Scholar]
- Canseco JA, Niklitschek EJ, Harrod C. 2021. Variability in δ13C and δ15N trophic discrimination factors for teleost fishes: a meta-analysis of temperature and dietary effects. Rev Fish Biol Fish 32: 1–17. [Google Scholar]
- Carlisle DM, Hawkins CP. 1998. Relationships between invertebrate assemblage structure, 2 trout species, and habitat structure in Utah mountain lakes. J North Am Benthol Soc 17: 286–300. [CrossRef] [Google Scholar]
- Closs GP. 2024. Salmonids in New Zealand: old ways in new lands. In Lobon-Cervia J, Budy P, Gresswell R eds. Advances in the Ecology of Stream-Dwelling Salmonids. Cham: Springer International Publishing 441–459. [CrossRef] [Google Scholar]
- Copp GH, Bianco PG, Bogutskaya NG, Eros T, Falka I, Ferreira MT, Fox MG, Freyhof J, Gozlan RE, Grabowska J, Kováč V, Moreno-Amich R, Naseka AM, Peňáz M, Povž M, Przybylski M, Robillard M, Russell IC, Stakenas S, Šumer S, Vila-Gispert A, Wiesner C. 2005. To be, or not to be, a non-native freshwater fish?. J Appl Ichthyol 21: 242–262. [CrossRef] [Google Scholar]
- Crawford SS, Muir AM. 2008. Global introductions of salmon and trout in the genus Oncorhynchus: 1870–2007. Rev Fish Biol Fish 18: 313–344. [CrossRef] [Google Scholar]
- Dejoux C, Iltis A. 1992. Lake Titicaca: A Synthesis of Limnological Knowledge, Dejoux C, Iltis A, eds., Kluwer Academic Publishers, Dordrecht, The Netherlands, 611 p. [Google Scholar]
- DeNiro MJ, Epstein S. 1977. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science 197: 261–263. [CrossRef] [PubMed] [Google Scholar]
- Dominguez E, Fernández HR. 2009. Macroinvertebrados bentónicos sudamericanos: sistemática y biología, Dominguez E, Fernández HR, eds., Fundación Miguel Lillo, Tucumán, 656 p. [Google Scholar]
- Dorador C, Pardo R, Vila I . 2003. Variaciones temporales de parámetros físicos, químicos y biológicos de un lago de altura: el caso del lago Chungará. Rev Chil Hist Nat 76: 15–22. [CrossRef] [Google Scholar]
- European Standard. 2005. Water quality Sampling of fish with multi-mesh gillnets. CEN TC 30 I.S.EN 14757:2005. [Google Scholar]
- Ferriz RA. 1994. Algunos aspectos de la dieta de cuatro especies ícticas del río Limay (Argentina). Rev Ichthyol 2: 1–7. [Google Scholar]
- Fry B. 2002. Stable isotopic indicators of habitat use by Mississippi River fish. J North Am Benthol Soc 21: 676–685. [CrossRef] [Google Scholar]
- Gannon J. 1976. The effects of differential digestion rates of zooplankton by alewife, Alosa pseudoharengus, on determinations of selective feeding. Trans Am Fish Soc 105: 89–95. [CrossRef] [Google Scholar]
- Gibson J. 1988. Mechanisms regulating species composition, population structure and production of stream salmonids: a review. Polish Arch Hydrobiol 35: 469–495. [Google Scholar]
- Guerrero CJ, Poulin E, Méndez MA, Vila I. 2015. Caracterización trófica de Orestias (Teleostei: Cyprinodontidae) en el Parque Nacional Lauca. Gayana 79: 18–25. [Google Scholar]
- González ER. 2003. The freshwater amphipods Hyalella Smith, 1874 in Chile (Crustacea: Amphipoda). Rev Chil Hist Nat 76: 623–637. [Google Scholar]
- Grey J, Thackeray SJ, Jones RI, Shine A. 2002. Ferox trout (Salmo trutta) as “Russian dolls”: Complementary gut content and stable isotope analyses of the Loch Ness foodweb. Freshw Biol 47: 1235–1243. [CrossRef] [Google Scholar]
- Harrod C, Grey J. 2006. Isotopic variation complicates analysis of trophic relations within the fish community of Plußsee: a small, deep, stratifying lake. Arch für Hydrobiol 167: 281–299. [CrossRef] [Google Scholar]
- Harrod C, Stallings CD. 2022. Trophodynamics. In Midway S, Hasler C, P C, eds. Methods for Fish Biology, American Fisheries Society, Bethesda, Maryland., 695–737. [Google Scholar]
- Harrod C, Grey J, McCarthy TK, Morrissey M. 2005. Stable isotope analyses provide new insights into ecological plasticity in a mixohaline population of European eel. Oecologia 144: 673–683. [CrossRef] [PubMed] [Google Scholar]
- Harrod C, Mallela J, Kahilainen KK. 2010. Phenotype-environment correlations in a putative whitefish adaptive radiation. J Anim Ecol 79: 1057–68. [CrossRef] [PubMed] [Google Scholar]
- Hembre LK, Megard RO. 2005. Timing of predation by rainbow trout controls Daphnia demography and the trophic status of a Minnesota lake. Freshw Biol 50: 1064–1080. [CrossRef] [Google Scholar]
- Herrera, C, Pueyo, JJ, Saez, A, & Valero-Garces, BL. (2006). Relation of surface and underground waters in Chungará and Cotacotani lake districts, northern Chile: an isotopic study. Revista Geológica de Chile 33: 299–325. [Google Scholar]
- Hubert WA, Gipson RD, McDowell RA, Stewart AC. 1994. Diet of Eagle Lake Rainbow Trout in Lake DeSmet, Wyoming. North Am J Fish Manag 14: 457–459. [CrossRef] [Google Scholar]
- Hyslop EJ. 1980. Stomach contents analysis a review of methods and their application. J Fish Biol 17: 411–429. [CrossRef] [Google Scholar]
- Jacobsen D, Dangles O. 2017. Ecology of high altitude waters, Oxford University press, 284 p. [Google Scholar]
- Jiménez-Seinos JL, Alcocer J, Planas D. 2023. Food web differences between two neighboring tropical high mountain lakes and the influence of introducing a new top predator. PLoS One 1–18. [Google Scholar]
- Kiljunen M, Grey J, Sinisalo T, Harrod C, Immonen H, Jones RI. 2006. A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models.J Appl Ecol 43: 1213–1222. [CrossRef] [Google Scholar]
- Knapp R, Matthews K, Sarnelle O. 2001. Resistance and resilience of alpine lake fauna to fish introductions. Ecol Monogr 71: 401–421. [CrossRef] [Google Scholar]
- Lobos G, Saez P, Villablanca R, Prado M, Cruz-jofré F, Fibla P. 2020. Invasion of salmonids in the Puna and Southern Chilean Altiplano: patterns and threats to the biodiversity. BioInvasions Rec 9: 853–864. [CrossRef] [Google Scholar]
- Logan JM, Jardine TD, Miller TJ, Bunn SE, Cunjak RA, Lutcavage ME. 2008. Lipid corrections in carbon and nitrogen stable isotope analyses: Comparison of chemical extraction and modelling methods. J Anim Ecol 77: 838–846. [CrossRef] [PubMed] [Google Scholar]
- Lowe S, Browne M, Boudjelas S, De Poorter M. 2000. 100 of the world’s worst invasive species A selection from the global invasive species database. The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), 12pp. First published as special lift-out in Aliens 12, December 2000. Updated and reprinted version: November., 105–110. [Google Scholar]
- Lynott ST, Bryan SD, Hill TD, Duffy WG. 1995. Monthly and size-related changes in the diet of rainbow trout in Lake Oahe, South Dakota. J Freshw Ecol 10: 399–407. [CrossRef] [Google Scholar]
- Macdonald A, Ruxton I. 2017. Trout Fishing in Chilean Rivers: A Concise Survey, Lulu.com, Morrisville, North Carolina, United States, 337 p. [Google Scholar]
- MacLennan MM, Vinebrooke RD. 2016. Effects of non-native trout, higher temperatures and regional biodiversity on zooplankton communities of alpine lakes. Hydrobiologia 770: 193–208. [CrossRef] [Google Scholar]
- Majdi N, Hette-Tronquart N, Auclair E, Bec A, Chouvelon T, Cognie B, Danger M, Decottignies P, Dessier A, Desvilettes C, Dubois S, Dupuy C, Fritsch C, Gaucherel C, Hedde M, Jabot F, Lefebvre S, Marzloff MP, Pey B, Peyrard N, Powolny T, Sabbadin R, Thébault E, Perga ME. 2018. There’s no harm in having too much: A comprehensive toolbox of methods in trophic ecology. Food Webs 17: e00100. [Google Scholar]
- Markevich G, Esin E. 2019. Trout and char of Russia. In: Kershner JL, Williams JE, Gresswell RE, Lobón-Cerviá J, eds.Trout and Char of the World, American Fishery Society., 777 p. [Google Scholar]
- Martín-Torrijos L, Sandoval-Sierra JV, Muñoz J, Diéguez-Uribeondo J, Bosch J, Guayasamin JM. 2016. Rainbow trout (Oncorhynchus mykiss) threaten Andean amphibians. Neotrop Biodivers 2: 26–36. [CrossRef] [Google Scholar]
- Matthews K, Knapp R. 1999. A study of high mountain lake fish stocking effects on the U.S. Sierra Nevada wilderness. Int J Wilderness 5: 24–26. [Google Scholar]
- McCutchan JH, Lewis WM, Kendall C, McGrath CC. 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102: 378–390. [CrossRef] [Google Scholar]
- Monroy M, Maceda-Veiga A, Caiola N, De Sostoa A. 2014. Trophic interactions between native and introduced fish species in a littoral fish community. J Fish Biol 85: 1693–1706. [CrossRef] [PubMed] [Google Scholar]
- Mouillet C, Barta B, Espinosa R, Andino P, Christoffersen KS, Jacobsen D. 2018. Ecological effects of introduced rainbow trout (Oncorhynchus mykiss) in pristine Ecuadorian high Andean lakes. Fundam Appl Limnol 191: 323–337. [CrossRef] [Google Scholar]
- Mühlhauser HA, Hrepic N, Mladinic P, Montecino V, Cabrera S. 1995. Water quality and limnological features of a high altitude Andean lake, Chungará, in northern Chile. Rev Chil Hist Nat 68: 341–349. [Google Scholar]
- Myers N, Mitter R, Mittermeier C, Fonseca G, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [CrossRef] [PubMed] [Google Scholar]
- Nannini M, Schermerhorn D, Wahl D. 2016. Lethal Stomach Content Collection Methods Reduce Detection of Zooplankton Prey of Juvenile Largemouth Bass Compared with Nonlethal Methods. North Am J Fish Manag 36: 738–743. [CrossRef] [Google Scholar]
- Nielsen JM, Clare EL, Hayden B, Brett MT, Kratina P. 2017. Diet tracing in ecology: Method comparison and selection. Methods Ecol Evol 9: 278–291. [Google Scholar]
- Northcote TG. 1997. Potamodromy in Salmonidae − living and moving in the fast lane. North Am J Fish Manag 17: 1029–1045. [CrossRef] [Google Scholar]
- O’Reilly CM, Hecky RE, Cohen AS, Plisnier PD. 2002. Interpreting stable isotopes in food webs: Recognizing the role of time averaging at different trophic levels. Limnol Oceanogr 47: 306–309. [CrossRef] [Google Scholar]
- Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O’Hara R, Solymos P, Stevens M, Simpson G, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista H, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill M, Lahti L, McGlinn D, Ouellette M, Ribeiro Cunha E, Smith T, Stier A,Ter Braak C, Weedon J. 2022. vegan: Community Ecology Package. R package version 2.6-4. [Google Scholar]
- Pardo R, Vila I. 2008. Threatened fishes of the world: Trichomycterus chungaraensis Arratia 1983 (Trichomycteridae). Environ Biol Fishes 81: 369–370. [CrossRef] [Google Scholar]
- Parnell AC. 2016. simmr: A stable isotope mixing model. Available at https://cran.r-project. org/web/packages/simmr/index.html. [Google Scholar]
- Pearse DE, Miller MR, Abadía-Cardoso A, Garza JC. 2014. Rapid parallel evolution of standing variation in a single, complex, genomic region is associated with life history in steelhead/rainbow trout. Proc R Soc B Biol Sci 281: 20140012. [CrossRef] [PubMed] [Google Scholar]
- Pérez PA, Docmac F, Harrod C. 2022. No evidence for effects of mill-grinding on δ13C, δ15N and δ34S values in different marine taxa. Rapid Commun Mass Spectrom 36: e9336. [CrossRef] [PubMed] [Google Scholar]
- Pethybridge H, Choy CA, Logan JM, Allain V, Lorrain A, Bodin N, Somes CJ, Young J, Ménard F, Langlais C, Duffy L, Hobday AJ, Kuhnert P, Fry B, Menkes C, Olson RJ. 2018. A global meta-analysis of marine predator nitrogen stable isotopes: Relationships between trophic structure and environmental conditions. Glob Ecol Biogeogr 27: 1043–1055. [CrossRef] [Google Scholar]
- Post DM. 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718. [Google Scholar]
- Pueyo JJ, Sáez A, Giralt S, Valero-Garcés BL, Moreno A, Bao R,Schwalb A, Herrera C, Klosowska B, Taberner C. 2011. Carbonate and organic matter sedimentation and isotopic signatures in Lake Chungará, Chilean Altiplano, during the last 12.3 kyr. Palaeogeogr Palaeoclimatol Palaeoecol 307: 339–355. [CrossRef] [Google Scholar]
- Quezada-Romegialli C, Jackson AL, Hayden B, Kahilainen KK, Lopes C, Harrod C. 2018. tRophicPosition, an R package for the Bayesian estimation of trophic position from consumer stable isotope ratios. Methods Ecol Evol 9: 1592–1599. [CrossRef] [Google Scholar]
- Quinn TP. 2018. The behavior and ecology of pacific salmon and trout, University of Washington Press; Bethesda,Maryland: In association with American Fisheries Society, Seatle, 508 p. [Google Scholar]
- R Core Team. 2023. R: A Languaje and Environment for Statistical Computing. [Google Scholar]
- Robinson BW, Wilson DS. 1994. Character release and displacement in fishes: A neglected literature. Am Nat 144: 596–627. [CrossRef] [Google Scholar]
- RStudio Team. 2023. RStudio: Integrated Development for R. [Google Scholar]
- Rundel PW, Palma B. 2000. Preserving the Unique Puna Ecosystems of the Andean Altiplano. Mt Res Dev 20: 262–271. [CrossRef] [Google Scholar]
- Sáez A, Valero-Garcés BL, Moreno A, Bao R, Pueyo JJ, González-Sampériz P, Giralt S, Taberner C, Herrera C, Gibert RO.2007. Lacustrine sedimentation in active volcanic settings: The Late Quaternary depositional evolution of Lake Chungará (northern Chile). Sedimentology 54: 1191–1222. [CrossRef] [Google Scholar]
- Schneidervin RW, Hubert WA. 1987. Diet Overlap among Zooplanktophagous Fishes in Flaming George Reservoir, Wyoming-Utah. North Am J Fish Manag 7: 397–385. [Google Scholar]
- Skúlason S, Smith T. 1995. Resource polymorphisms in vertebrates. Trends Ecol Evol 10: 366–370. [CrossRef] [PubMed] [Google Scholar]
- Smith CW. 1985. Impact of alien plants on Hawaii. In: Stone CP,Scott JM, eds. Hawaii terrestrial ecosystems: preservation and management, University of Hawaii Cooperative National Park Resources Studies Unit, Honolunu., 180–250. [Google Scholar]
- Swanson HK, Lysy M, Power M, Stasko AD, Johnson JD, Reist J. 2015. A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 96: 318–324. [CrossRef] [PubMed] [Google Scholar]
- Swynnerton GH, Worthington EB. 1940. Notes on the Food of Fish in Haweswater. In M. H. Engel, &S. A. Macko (Eds.) O geochemistry (pp. 73–98). NYS, ed. J Anim Ecol 9: 183–187. [CrossRef] [Google Scholar]
- Taylor EB. 1999. Species pairs of north temperate freshwater fishes: Evolution, taxonomy, and conservation. Rev Fish Biol Fish 9: 299–324. [CrossRef] [Google Scholar]
- Thomas SM, Crowther TW. 2015. Predicting rates of isotopic turnover across the animal kingdom: A synthesis of existing data. J Anim Ecol 84: 861–870. [CrossRef] [PubMed] [Google Scholar]
- Tiberti R, von Hardenberg A, Bogliani G. 2014. Ecological impact of introduced fish in high altitude lakes: A case of study from the European Alps. Hydrobiologia 724: 1–19. [CrossRef] [Google Scholar]
- Vander Zanden MJ, Cabana G, Rasmussen JB. 1997. Comparing trophic position of freshwater fish calculated using stable nitrogen isotope ratios (δ15N) and literature dietary data. Can J Fish Aquat Sci 54: 1142–1158. [CrossRef] [Google Scholar]
- Vander Zanden MJ, Casselman JM, Rasmussen JB. 1999. Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 401: 1997–2000. [Google Scholar]
- Vila I, Pinto M. 1986. A new species of killifish (Pisces, Cyprinodontidae) from the Chilean Altiplano. Rev d’Hydrobiologie Trop 19: 233–239. [Google Scholar]
- Vila I, Pardo R, Scott S. 2007. Freshwater fishes of the Altiplano. Aquat Ecosyst Heal Manag 10: 201–211. [CrossRef] [Google Scholar]
- Vimos DJ, Encalada AC, Ríos-Touma B, Suárez E, Prat N. 2015. Effects of exotic trout on benthic communities in high-Andean tropical streams. Freshw Sci 34: 770–783. [CrossRef] [Google Scholar]
- West JB, Bowen GJ, Cerling TE, Ehleringer JR. 2006. Stable isotopes as one of nature’s ecological recorders. Trends Ecol Evol 21: 408–414. [CrossRef] [PubMed] [Google Scholar]
- Wilcomme R. 1988. International introductions of inland aquatic species. FAO, Rome, Fish Tech Pap 294, 328 p. [Google Scholar]
- Winemiller KO. 1990. Spatial and Temporal Variation in Tropical Fish Trophic Networks. Ecol Monogr 60: 331–367. [CrossRef] [Google Scholar]
Cite this article as: González K, Rivara P, Docmac F, Gomez−Uchida D, Harrod C. 2024. Habitat-based variation in the trophic ecology of the world’s highest-altitude self-sustaining population of invasive rainbow trout. Knowl. Manag. Aquat. Ecosyst., 425, 18.
All Tables
Results of linear regression of individual liver stable isotope values (y) on muscle stable isotope values (x) for rainbow trout captured in the three different habitats sampled in Chungará catchment. Errors are ± 1 S.E.
Results of Pearson’s correlation tests examining relations between rainbow trout size (FL, mm) and stable isotope values. Results are shown separately for liver and muscle tissues and for fish captured from the lake, the river mouth, and the river in the Chungará catchment.
All Figures
 |
Fig. 1 Location of Lake Chungará in A) South America and B) Chile. The relative location of the different sampling habitats described in the text are shown. |
| In the text | |
 |
Fig. 2 Histogram showing the fork length (FL) in mm of rainbow trout caught in the lake (gillnet, n = 37), river mouth (electrofishing, n = 18) and river (electrofishing, n = 91). |
| In the text | |
 |
Fig. 3 NMDS ordination biplot (2d stress = 0.13) based on Bray-Curtis dissimilarity showing variation in NMDS 1 and 2 scores based on the contents of individual rainbow trout stomachs for fish sampled in three different habitats. Each point reflects a single individual, and points located closer together have stomach contents that are more similar than those further away. Significant taxa in the diet (those explaining most of the variance among fish) and their vector loadings are indicated in black. Note the trophic segregation between capture habitats (i.e. lake, river mouth, and river trout all fall into distinct ordination space) and the reduced inter-individual variation shown by lake fish in comparison to the other habitats. |
| In the text | |
 |
Fig. 4 Stable isotope A) δ15N − δ13C, B) δ15N − δ34S and C) δ13C − δ34S biplots showing variation in individual values for rainbow trout liver (brown filled circles), muscle (orange filled circle), and putative prey (filled triangles) from different habitats. Note that these are data uncorrected for trophic discrimination, and that δ13C values for rainbow trout (but not their prey) have been corrected for lipid effects. |
| In the text | |
 |
Fig. 5 Box plots comparing the distribution of carbon, nitrogen, and sulphur stable isotope values from liver (dark brown) and muscle (orange) tissue from rainbow trout captured from the lake, river mouth and river of Chungará catchment. Solid horizontal lines represent the median values; boxes represent the interquartile range (IQR) and whiskers represent the variability beyond the IQR (i.e. Q1–1.5*IQR, Q3 + 1.5*IQR). T-test results reflect the significance of pairwise comparisons of each isotope values at individual level from liver and muscle tissue in each habitat (one sample t-test). |
| In the text | |
 |
Fig. 6 Visual representation of simmr mixing model results by habitat and tissue type. Boxplots showing the distribution of posterior estimates of the contribution of the different putative prey to assimilated diet to rainbow trout from the lake (A: liver, B: muscle), river mouth (C: liver, D: muscle), and river (E: liver, F: muscle). Note that the dietary categories differ between habitats. Solid horizontal lines represent the median values; boxes represent the interquartile range (IQR), and whiskers represent the variability beyond the IQR (i.e. Q1–1.5*IQR, Q3 + 1.5*IQR). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


