| Issue |
Knowl. Manag. Aquat. Ecosyst.
Number 425, 2024
Anthropogenic impact on freshwater habitats, communities and ecosystem functioning
|
|
|---|---|---|
| Article Number | 2 | |
| Number of page(s) | 12 | |
| DOI | https://doi.org/10.1051/kmae/2023026 | |
| Published online | 23 January 2024 | |
Research Paper
Distribution of the invasive polychaete Hypania invalida (Grube, 1860) against the background of the benthic fauna in the upper Oder River catchment (Poland)
Institute of Biology, Biotechnology and Environmental Protection, Faculty of Natural Sciences, University of Silesia, Bankowa 9, 40-007 Katowice, Poland
* Corresponding author: mariola.krodkiewska@us.edu.pl
Received:
11
July
2023
Accepted:
21
December
2023
Biological invasions are one of the greatest threats to biodiversity, economic development, and human health. Therefore an important challenge is to understand the mechanisms and factors that facilitate the spread of invasive species. The Ponto-Caspian polychaete Hypania invalida is one of the invaders that have been colonizing the river systems in Europe since the 1950s. The research aim was to map the distribution of H. invalida in the upper Oder and associated aquatic environments, to assess the contribution of H. invalida to the benthic fauna, to identify environmental factors linked to the occurrence of H. invalida, and to provide genetic markers that can be used to identify H. invalida and monitor its dispersal. H. invalida was found at two sites in the canalized section of the upper Oder and one site at the initial section of the Gliwice Canal. It was found at low abundance and coexisted with other alien macroinvertebrates. H. invalida was not recorded in any smaller river, whether natural, semi-natural, or anthropogenically modified. Apart from the watercourse width, no significant relationships between this polychaete and habitat drivers were found. Genetic analysis showed that the amplified cytochrome c oxidase subunit I and 18S gene fragments showed no sequence variation across all analyzed specimens. Further research is needed to follow the spread of H. invalida in the upper Oder River catchment, as at high densities it can adversely affect inhabited ecosystems.
Key words: Alien species / Polychaeta / inland waters / DNA barcoding
© M. Krodkiewska et al., Published by EDP Sciences 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
1 Introduction
Biological invasions are one of the greatest threats to global biodiversity, economic development, and human health (Pyšek et al., 2020). Inland waters, especially river systems, are particularly vulnerable to invasions due to their location in the landscape, high levels of connectivity, and human usage (Olden et al., 2021). The assessment and management of invasive species is difficult and costly (Cuthbert et al., 2021), so it is crucial to understand the mechanisms and factors that facilitate their expansion in aquatic ecosystems.
The polychaete Hypania invalida (Grube, 1860) is one of the invaders that have colonized many river systems in Europe since the 1950s (Bij de Vaate et al., 2002; Gherardi et al., 2009). This Ponto-Caspian species used three corridors of migration described by Bij de Vaate et al. (2002): the Northern (Volga basin), the Central (Dnieper and Vistula basins), and the Southern (Danube basin), to spread in European waters (Fig. 1). The Danube River catchment has played a crucial role in the dispersion of H. invalida (Pavel et al., 2021), allowing its colonization in numerous river systems across Western and Central Europe. The species has settled in the Rhine, the Main (Schmidt et al., 1998; Tittizer et al., 2000), the Elbe, the Havel, the Moselle (Tittizer et al., 2000; Eggers and Anlauf, 2008; Straka et al., 2015; Müller et al., 2018), the Meuse (Vanden Bossche et al., 2001; Vanden Bossche, 2002), the Seine, the Marne, the Rhone (Devin et al., 2006; Bij de Vaate et al., 2007; Besacier-Monbertrand et al., 2014), the Sava (Zorić et al., 2011; Vučković et al., 2021), the Drava (Vučković et al., 2021), the Tisa (Ferencz, 1969; Szító, 1996; Zorić et al., 2011), and the Oder rivers (Wozniczka et al., 2011; Pabis et al., 2017). It has also been found in various canals linking river systems (Tittizer et al., 2000; Müller et al., 2006). Beyond continental Europe, it was found in the Thames in England in 2008 (Gallardo and Aldridge, 2013) (Fig. 1). The expansion of H. invalida in inland waters has been made easier by the rapid upstream movement of juveniles through ballast water of ships. Additionally, natural passive drift, especially during floods, has increased downstream spread (Bij de Vaate, 2003; Norf et al., 2010; Wozniczka et al., 2011). In the future, it cannot be ruled out that H. invalida will be introduced to waterways in North America through transport in ballast water, like many other macroinvertebrates of Ponto-Caspian origin. However, current ballast water regulations are likely to hinder this process (ballast water exchange, 30 parts per thousand (ppt) flushing) (Baker et al., 2023).
H. invalida is a eurytopic species that can be found in a wide range of environmental conditions. It is one of the few polychaetes that inhabits freshwater (Glasby and Timm, 2008), but it can also tolerate waters with salinities up to 12 ppt (Mordukhai-Boltovskoi, 1964; Norf et al., 2010). It can tolerate a wide range of temperatures (2–25°C), depths (from the nearshore to above 900 m) (Norf et al., 2010), water with alkaline pH (Vanden Bossche et al., 2001), and various substrates (Filinova et al., 2008; Norf et al., 2010).
H. invalida exhibits many characteristics of an invasive species, such as tolerance of various habitat conditions, ease of dispersal, early sexual maturity, rapid growth, high fecundity, high reproductive potential, and a broad diet. It is an active filter and deposit feeder with a non-specific food preference (Bij de Vaate et al., 2002), feeding primarily upon diatoms (Gruia and Manoleli, 1974; Manoleli, 1975). These traits have led to its recognition as an invasive species that can cause changes in the food web of colonized aquatic ecosystems (Norf et al., 2010). Thus far, however, no negative impact of H. invalida on the functioning of newly inhabited ecosystems has been demonstrated (Devin et al., 2006; Vučković et al., 2021). Nevertheless, if its populations reach very high densities, some effects may be expected (Straka et al., 2015). It should be noted that this polychaete can improve water quality by reducing the amount of organic matter in the water and sediment. It can also promote the activity of aerobic bacteria by aerating the sediment through bioturbation (Surugiu, 2005). H. invalida builds tubes made of sand and detritus and can change substrates through such a lifestyle (Pavel et al., 2021). It can also serve as food for various fish species, e.g. bream, white bream, and round goby (Băcescu and Dumitrescu, 1958; Yakovlev and Yakovleva, 2010; Shcherbina, 2012).
To date in Poland, H. invalida has only been recorded in the Oder River. It has been found in the estuary area (Wozniczka et al., 2011) and the upper reaches of the river (Pabis et al., 2017). Taking the above findings into account, the aims of our research were: (1) to map the distribution of H. invalida in the upper Oder and associated aquatic environments, (2) to assess the contribution of H. invalida to the benthic fauna, (3) to identify environmental factors linked to the occurrence of H. invalida, (4) to provide genetic markers that can be used to identify H. invalida and monitor its dispersal.
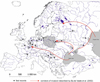 |
Fig. 1 The first records of H. invalida in European rivers outside the area of natural occurrence based on reference data. Abbreviation: sof references: 1Popescu-Marinescu (1992), 2Semenchenko et al. (2016), 3Ketelaars (2004), 4Dzyuban and Slobodchikov (1980), 5Ferencz (1969), 6Zorić et al. (2011), 7Lvova et al., (1996), 8Klink and Bij de Vaate (1996), 9Tittizer et al. (2000), 10Tischikov and Tischikov (2005), 11Vanden Bossche et al. (2001), 12Devin et al. (2006), 13Eggers and Anlauf (2008), 14Gallardo and Aldridge (2013), 15Wozniczka et al. (2011), 16Müller et al. (2018), 17Vučković et al. (2021). |
2 Materials and methods
2.1 Study area
The study was carried out along a stretch of the upper Oder River between 108 and 85 km, in the Gliwice and Kłodnica canals, which are related to the upper Oder River, and in the Kłodnica River and its tributaries (Fig. 2).
The Oder River is Poland’s second-longest river and an important transborder river in Central Europe. It flows through the Czech Republic, Poland and Germany. The length of the Oder is 854 km (742 km in Poland), and it drains an area of 118 861 km2 (106 056 km2 in Poland). The river has been significantly altered by human activities over the decades, including regulatory and reservoir developments in its upper and middle reaches, and its connection to other European river systems via the Oder-Havel and Oder-Spree canals.
The Gliwice Canal connects the port on the Oder River in Kedzierzyn-Kozle to the inland port in Gliwice. It is 41.2 km long and has a difference in level of 43.6 m between the beginning and end of the canal. The bottom of the canal is covered with a layer of clay and the banks are reinforced with a bed of stones. The canal is mainly fed by the Kłodnica and Drama rivers. The Gliwice Canal plays an important role in the Oder River Waterway, which is the most significant waterway for shipping and cargo transport in Poland, connecting the port of Szczecin and Western Europe via the Oder River and Germany’s inland waterways (Kozerska, 2016).
The Kłodnica Canal linked the industrial Upper Silesia with the Oder River but was closed in the 1930s after the opening of the Gliwice Canal. Nowadays, only 3.5 km of the canal remains and it serves as both a technical monument and tourist attraction. The Kłodnica River feeds it.
The Kłodnica River is a right tributary of the upper Oder River, discharging into the Oder at 94 km. It has a length of 84 km and a catchment area of 1126 km2. The upper reaches of the river flow through heavily urbanized and industrialized regions, while the further course of the river drains mostly rural areas. The river is heavily polluted with industrial and municipal waste, including saline waste from coal mines and runoff from industrialized and urban areas (Olkowska et al., 2014).
The selected tributaries of the Kłodnica River (Fig. 2) exhibit varying degrees of anthropogenic impact. The lower and middle course tributaries are predominantly fed surface runoff from agricultural areas. They are mostly streams with natural, unregulated channels. In contrast, the tributaries in the upper reach of the Kłodnica River flow through densely populated and industrial areas of the Upper Silesian Coal Basin and are anthropogenically transformed, their beds being strengthened and often concreted.
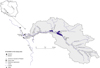 |
Fig. 2 Area of the study. Abbreviations of study sites: the Oder River (O1-O3), the Gliwice Canal (KG1-KG11), the Kłodnica Canal (KK), the Kłodnica River (K1-K17), tributaries of the Kłodnica River (M-Młynówka Stream, Jo-Jordan Stream, PT-Toszecki Stream, D-Drama River, B-Bytomka River, C-Czerniawka River, PB-Bielszowicki Stream, P-Promna Stream, Ja-Jamna Stream, S-Ślepiotka Stream). |
2.2 Methods
2.2.1 Field and laboratory study
The material for the study was collected at 42 locations, i.e. 3 sites in the Oder River, 11 sites in the Gliwice Canal, 1 site in the Kłodnica Canal, 17 sites in the Kłodnica River, and 10 sites in the Kłodnica River tributaries. Each locality was sampled in the summer of 2017 and 2018. Apart from the Order River, at all sites samples were only taken from the riverbank using a cuboid frame (frame dimensions: W 25 × D 25 × H 50 cm) out of which stones were manually removed, and invertebrates were brushed off into a plastic container. The residual material within the frame, approximately 5 cm in depth, was collected using a 500 µm mesh and transferred to a container. At all sites in the Oder River, two samples were taken, one from the riverbank using a cuboid frame and the other at a distance of approximately 2 m from the riverbank using an Ekman grab (surface area: 16 × 16 cm). At the Oder sites, one sample was created by combining material from three Ekman grabs (collection area = 768 cm2 of bottom) or three frame samples (collection area = 1875 cm2 of bottom). The collected samples were sieved through a 500 µm mesh sieve in the laboratory and stored in 80% ethanol. The macroinvertebrates were separated from the sediments under a dissection microscope, identified to the lowest possible level, and enumerated.
During the field work, water parameters, such as water conductivity, total dissolved solids, salinity, pH, temperature, oxygen and oxygenation, were measured at each sampling site using HI 9811‐5 Hanna Instruments and Multi 3410 WTW portable meters. The laboratory meters from Hanna Instruments and Merck were used to test other water parameters such as chlorides, sulfates, potassium, nitrates, nitrites, ammonium and phosphate content, total hardness, and alkalinity. These were tested following standard methods (Hermanowicz et al., 1999). Sediment samples from all sites were collected to assess the organic matter content and grain size composition of the bottom sediments. The total organic matter content (%) in the sediments was determined using the loss-on-ignition technique by combusting them at 550 °C for 4 h (Myslinska, 2001). The grain size composition of the sediments was examined using the sieve method according to the Polish standard (PKN‐CEN ISO/TS 17892‐4, 2009). The water velocity was measured using the float method in the canals, the Kłodnica River, and its tributaries (Dobriyal et al., 2017). Data on water velocity in the Oder River were obtained from the Institute of Meteorology and Water Management − National Research Institute (IMWM‐NRI, 2020). Depth was measured at all sampling sites.
2.2.2 DNA barcoding
The material for molecular studies consisted of 17 individuals collected from two locations (eight individuals from the Odra River and nine individuals from the Gliwice Canal). Pieces of the anterior and posterior parts of the body were used for DNA isolation, avoiding fragments with remnants of gut contents. The remaining parts of the individuals were preserved in a 96% ethanol-filled tube as a backup and stored at −20°C. DNA isolation was performed using a column method with proteinase K and RNase A digestion step (GeneMATRIX Tissue DNA Purification Kit) (EURx, Gdansk, Poland) according to the manufacturer's protocol. Fragments of two commonly used marker genes, mitochondrial cytochrome c oxidase subunit I (COI) and nuclear 18S rRNA (18S), were amplified. COI amplification was amplified on all 17 individuals, while 18S was analyzed on seven individuals from the same pool. Polymerase chain reactions (PCRs) were carried out in 50 μL reactions containing 21 μL of double-distilled water, 25 μL of Color OptiTaq PCR Master Mix (2x) (EURx, Gdansk, Poland), 1 μL of each primer at a 10 mM concentration, and 1 μL of total genomic DNA as a template. For COI amplification, the LCO1490 and HCO2198 primer set was used (Folmer et al., 1994). Initially, we attempted to amplify 18S as two partially overlapping segments using primer A combined with Rev1044a18S for “segment A” and Frw921b18S combined with primer B for “segment B” (Apakupakul et al., 1999; de Carle et al., 2022). However, this approach, usually successful for other annelids, produced low-quality reads for H. invalida. Therefore, we designed and tested a series of new primer sets based on the consensus sequence assembled with those low-quality reads. Finally, the 18S was successfully amplified and sequenced using Hyp18s_54F combined with Hyp18s_1135R and Hyp18s_833F combined with Hyp18s_1951R, which allowed us to obtain high-quality reads for the gene. The amplification products were sent to GenoMed (Warsaw, Poland) and sequenced in both directions. Table 1 lists all effective primers and thermal cycling profiles.
Primers and thermal cycling profiles used for DNA barcoding of H. invalida.
2.2.3 Statistical analysis
To describe the structure of the benthic fauna, the abundance of taxa and their contribution to total zoobenthos were calculated. Ordination statistics were conducted using the program Canoco Version 5.0 (Šmilauer and Lepš, 2014). Analyses were carried out on a biological data matrix (density of alien species and density of other macroinvertebrates identified to the family level) and an environmental data matrix. The results of the preliminary analysis (detrended correspondence analysis) indicated that the variation in the biological data was best described by an unimodal model (the length of the gradient was 3.198). Therefore, a canonical correspondence analysis (CCA) was used to estimate the relative importance of environmental variables for biological data. First, a CCA with a stepwise forward selection (with Monte Carlo permutations = 499 runs) including all environmental variables (water parameters, granulometric composition of sediments, content of organic matter in sediments, current velocity, width, depth, nature of the river section, i.e. natural section or hydromorphologically modified section) was conducted to select the variables that explained most of the variability in macroinvertebrate density. The Pearson product-moment correlations were then calculated among the selected environmental variables to check for redundancy. Rare taxa (those that occurred in less than 5% of samples) were removed from the analysis to reduce noise in the data set (Gauch, 1982). After removing the rare taxa and redundancy among the significant environmental variables (chlorides and sulfates were excluded from the analysis because they were correlated with conductivity), 10 environmental variables and 36 taxa were used in the final CCA. The explanatory variables included conductivity, current velocity, width, the content of organic matter in sediments, %stone, %gravel, % sand, %silt, and the character of the river stretch (natural versus hydromorphologically modified). Analyses were conducted on log (x + 1)-transformed invertebrate density and environmental data. The results were presented graphically in an ordination diagram.
Multiple regression analysis (backward variable elimination) was used to assess the relationship between environmental variables and the contribution of alien species to the zoobenthos community. With each step in a regression, the environmental variable with the lowest partial effect indicated by the highest p-value was removed until only environmental variables related (p ≤ 0.05) remained. This analysis was carried out using Statistica ver. 13.1.
3 Results
3.1 Habitat characteristics
The characteristics of the habitat parameters of the studied environments are presented in Table 2. The salinity and salinity-related parameters such as chlorides, sulfates, potassium, and total hardness were elevated in the Oder River and both canals. In contrast, these parameters exhibited substantial variation in the Kłodnica River and its tributaries (Tab. 2). High values were recorded along the entire course of the Kłodnica River, except for the river's source stretch, and in the tributaries upstream. In contrast, salinity-related parameters were low in the tributaries in the middle and lower reaches of the Kłodnica River.
The pH of water at all sites was either neutral or slightly alkaline. The concentrations in the water of nitrite, nitrate, ammonium and phosphate varied considerably at the sampling sites in all watercourses. They were usually higher in the upper Kłodnica and its upper tributaries. The sediments in the upper reaches of the Kłodnica River and these tributaries were characterized by a higher content of the fine-grained fraction and content of organic matter than in other environments.
Water flow velocity in the Gliwice and Kłodnica canals was lower than in other environments (Tab. 2).
Characteristics of the sampling sites in the studied area.
3.2 Distribution of H. invalida in the study area
In the study area, the polychaete H. invalida was exclusively found in three localities, i.e. two sites in the canalized section of the upper Oder River (site O1 at 108 km and site O2 at 95.3 km) and one site in the initial section of the Gliwice Canal (site KG1) (Fig. 2). At site O1, in the area up to a depth of 1 m, it reached densities of 16 individuals/m2 to 171 individuals/m2 on sandy substrate, and at depths above 1 m it achieved densities of 800 individuals/m2 to 1133 individuals/m2 on muddy sediments. At site O2, the density of H. invalida ranged from 15 individuals/m2 to 2030 individuals/m2 on a silty-sandy substrate. At the Gliwice Canal site (site KG1), its density was 176 individuals/m2 on the bottom, which was covered by a thick layer of live zebra mussels and empty shells (Tab. 3).
H. invalida individuals accounted for up to 13.2% of the total benthic macroinvertebrate abundance in the benthic invertebrate communities (Tab. 4). Other non-native macroinvertebrates were co-occurring with H. invalida (Tab. 4). These included the polychaete Laonome xeprovala (Bick, Bastrop 2018), the amphipods Dikerogammarus villosus (Sowinsky, 1894) and Gammarus tigrinus (Sexton, 1939), the gastropod Potamopyrgus antipodarum (Gray, 1843), and the bivalves Corbicula fluminea (Müller, 1774), and Dreissena polymorpha (Pallas, 1771) at all sites. The contribution of non-native species to the total fauna was high, and especially very high at one site in the Oder River (site O1) and one in the Gliwice Canal (site KG1) (Tab. 4). Beside alien species, only the native brackish amphipod Apocorophum lacustre (Vanhőffen, 1911) was abundant at all these sites (Tab. 3).
In the CCA for all studied sites, which included only the environmental variables determined by the forward selection (conductivity, current velocity, width, the content of organic matter in sediments, %stone, %gravel, %sand, %silt, and nature of the river stretch), the first two axes explained 46.6% of the variability in the data on the composition of the benthic fauna. The relationship between the data of the macroinvertebrate fauna and the explanatory environmental variables accounted for 74.1%. The Monte Carlo permutation tests showed that the CCA's ordination axes were significant (Tab. 5). The most alien species, except for the two oligochaetes P. bavaricus and P. albicola, were associated with larger streams of altered hydromorphology than with smaller natural streams. Along the first axis, the main gradient of change in benthic invertebrate composition, including the abundance of H. invalida, was related to river width (Fig. 3). The second ordinate axis was related to the gradient of salinity parameters (conductivity, chloride and sulphate content), as salinity increased, the density of alien species as P. antipodarum, P. acuta, D. polymorpha and G. tigrinus increased (Fig. 3). Regression analysis showed that the share of alien species in the macroinvertebrate community was positively related to salinity-related parameters, e.g. conductivity, chlorides and sulphates (adj. R2 = 0.58597, p <0.0001).
At sites in the Oder River, empty tubes and tubes containing H. invalida formed a thin layer of silt on coarse-grained sediments, which was often colonized by other benthic macroinvertebrates, e.g. mollusks and amphipods (Fig. 4).
Abundance of macroinvertebrates (per m2) at the sites where H. invalida was found.
Relative abundance (%) of alien versus native macroinvertebrates in benthic fauna at the sites where H. invalida occurred.
Summary of canonical correspondence analysis (CCA) performed on macroinvertebrate data and environmental variables determined by forward selection.
 |
Fig. 3 Ordination diagram based on the canonical correspondence analysis of the taxa abundance data and the best explanatory variables. Abbreviations: alien species Ch.cur-Ch.curvispinum, C.flu-C.fluminea, D.hae-D.haemobaphes, D.pol-D.polymorpha, D.vill-D.villosus, G.tig-G.tigrinus, H.inv-H.invalida, L.xep-L.xeprovala, P.acu-P.acuta, P.bar-P.barbatus, P.bav-P.bavaricus, P.alb-P.albicola, P.ant-P.antipodarum, other macroinvertebrate taxa Asel-Asellidea, Baet-Baetidae, Bith-Bithyniidae, Caen-Caenidae, Cera-Ceratopogonidae, Chiro-Chironimidae, Coen-Coenagrionidae, Coro-Corophidae, Dyti-Dytiscidae, Ecno-Ecnomidae, Ench-Enchytraeidae, Erpo-Erpobdellidae, Gloss-Glossiphoniidae, Hali-Haliplidae, Hydr-Hydropsychidae, Lymn-Lymnaeidae, Meso-Mesoveliidae, Naid-Naididae, Psycho-Psychodidae, Simu-Simuliidae, Spha-Sphaeriidae, Veli-Veliidae, Vivi-Viviparidae, environmental variables Mod-hydromorphologically modified river stretch, Nat-natural river stretch |
 |
Fig. 4 (A) Sediment with tubes of H. invalida from the upper Oder River; (B) Hypania invalida. |
3.3 DNA barcoding
As a result of molecular analysis, sequences for the COI and 18S genes of H. invalida were acquired. The study did not reveal any differences among specimens at the analyzed loci. The generated sequences were deposited in the GenBank database under accession numbers MK757472 and MT019843, respectively.
4 Discussion
4.1 Distribution of H. invalida and environmental conditions
Our research has revealed the upstream dispersion of H. invalida along the Oder River and to the initial section of the Gliwice Canal. Previous research identified its presence at 108 km in the Oder (Pabis et al., 2017). Two years later, we detected this polychaete at 95.3 km, confirming its potential to move upstream. The rate of H. invalida spread up the Oder may have been aided by the river's use for shipping and cargo transport (Maruszczak, 2019), particularly since juveniles can easily be transported in ballast water. Another factor that may have facilitated the spread of this euryhaline polychaete species (Norf et al., 2010) is the increasing water salinity of the Oder River. The elevated salinity of the upper reaches of the river is caused by several factors, including the discharge of underground saline water from the Upper Silesian Coal Basin's coal mines into its tributaries such as the Kłodnica River (Matysik, 2019). Salt-tolerant alien species often colonize environments with higher salinity, where native freshwater species cannot thrive (Grabowski et al., 2007).
We observed a higher population density of H. invalida (up to 2030 individuals/m2) compared to the study by Pabis et al. (2017), which only found singular individuals in the upper Oder River. However, these densities were not as high as those found in other localities such as the Szczecin Lagoon (up to 11 466 individuals/m2) (Wozniczka et al., 2011), the lower section of the Danube River (46 875 individuals/m2 in 1072 km at Baziaş, Romania) (Pavel et al., 2021), or the lower part of the Moselle River (up to 11 431 individuals/m2 at Sierck-les-Bains, France) (Devin et al., 2006).
Our findings indicate that H. invalida is mainly associated with the stretch of the Oder River that has been hydromorphologically modified for shipping and cargo transport. Outside the Oder, it was only found in the initial section of the Gliwice Canal. Thus, our study aligns with previous research (Zorić et al., 2011), suggesting that H. invalida is widely distributed in rivers undergoing hydromorphological changes and affected by heavy shipping traffic. However, Vučković et al. (2021) pointed out that this species also inhabits natural river sections and smaller channels in Croatia and suggested that the migration of H. invalida into such environments could occur through the use of fishing nets by anglers.
Our research found no significant correlations between the occurrence of H. invalida and the studied factors except for the watercourse width, the parameter that is determined by Strahler stream order, slope and land use, among others (Downing et al., 2012). Previous research indicates that H. invalida is more commonly found in high densities in environments with low-flow conditions where sedimentation rates are high, and rarely in environments with high-flow velocities, where coarse-grained sediments prevail (Băcescu, 1949; Devin et al., 2006; Filinova et al., 2008; Wozniczka et al., 2011).
The finding of H. invalida in the Gliwice Canal on a bottom covered with a thick layer of live D. polymorpha and empty shells confirms the results of Shcherbina (2001), Norf et al. (2010) and Yakovlev and Yakovleva (2010), who reported that this alien polychaete often inhabits dreissenid druses (D. polymorpha and D. bugensis), which provide it with shelter, tube-building material and food (Straka et al., 2015).
Our study, like the earlier research by Klink and Bij de Vaate (1996), Vanden Bossche et al. (2001), and Pavel et al. (2021), showed that H. invalida coexists with other alien macroinvertebrates from the Ponto-Caspian region, such as the oligochaetes Potamothrix bavaricus (Oschmann, 1913), Potamothrix moldaviensis Vejdovský and Mrázek, 1903, Psammoryctides barbatus (Grube, 1860), and Psammoryctides albicola (Michaelsen, 1901) (Dumnicka, 2016), the amphipods Chelicorophium curvispinum (G.O. Sars, 1895), Dikerogammarus haemobaphes (Eichwald, 1841), and Dikerogammarus villosus (Sovinsky, 1894), and the bivalve Dreissena polymorpha.
Taking into account the low population density of H. invalida in the study area, it can be supposed that it has currently no impact on the benthic fauna. It is worth noting that the effect of H. invalida on colonized ecosystems outside its natural range has not yet been fully recognized. Zorić et al. (2011) suggested that H. invalida had limited influence on benthic assemblages due to its preference for soft sediment, and Devin et al. (2006) found that it was a weak competitor for environmental resources. Nevertheless, at high densities, the impact of this polychaete on the ecosystem cannot be ruled out (Straka et al., 2015). With global climate change, shorter periods of ice cover and higher water temperatures may extend the breeding season of H. invalida (Norf et al., 2010), potentially contributing to an increase in its population size. Thus, further research is necessary to evaluate the population changes of this euryhaline polychaete and its future spread in the upper Oder River.
4.2 Molecular data
Although amplifying and sequencing COI for H. invalida were straightforward, obtaining the 18S rDNA barcode required additional effort. However, our persistence paid off, and we obtained reliable sequences for both genes. We believe that the newly designed primer sets used to amplify the 18S rRNA gene could be useful for other researchers monitoring the spread of H. invalida. We deposited the generated sequences in the GenBank database in 2020. However, it is surprising that, to date, no additional marker genes, mitochondrial haplotypes, or nuclear haplotypes for this invasive species have been publicly deposited in databases. Other marker gene sequences for H. invalida, obtained from Sanger sequencing and RNA-Seq data, can be found in the supplementary data provided in the paper by Stiller et al. (2020), who analyzed specimens collected in Magdeburg, Germany. Although the shorter partial COI sequence retrieved from that data was identical to our Polish haplotype (MK757472), the 18S sequence was missing, perhaps due to Sanger sequencing issues similar to the ones we encountered. However, mitochondrial 16S rDNA, nuclear 28S rDNA, and histone H3 sequences were provided based on transcriptomic data. H. invalida is currently defined by five commonly used marker genes, although this information may not be widely known. We encourage researchers studying H. invalida to deposit their data in publicly accessible databases to facilitate knowledge sharing and advance research on this important invasive species. The identical COI sequence of specimens from the Oder and Elbe in Magdeburg supports our suggestion that the Oder population may originate from the Elbe population, which was discovered early (Eggers and Anlauf, 2008), or from riverine ecosystems linked to the Elbe, given its connection to the Oder via the Elbe-Havel and Havel-Oder Canals. However, this hypothesis is based on limited evidence. Before any definitive conclusions can be drawn, more molecular studies are necessary to analyze the genetic diversity of H. invalida individuals from various locations in Europe and to determine whether there are any significant genetic differences between populations.
Acknowledgements
The authors thank Krzysztof Pabis for his help with the identification of Hypania invalida. The authors thank the Translmed Publishing Group (Cedar Hill, TX, USA) and Richard Ashcroft for correcting and improving the language of the manuscript.
References
- Apakupakul K, Siddall ME, Burreson EM. 1999. Higher level relationships of leeches (Annelida: Clitellata: Euhirudinea) based on morphology and gene sequences. Mol Phylogenet Evol 12: 350–359. [CrossRef] [PubMed] [Google Scholar]
- Baker E, Dettloff K, Bartos A. 2023. Hypania invalida (Grube, 1860): U.S. Geological Survey, Nonindigenous Aquatic Species Database, Gainesville, FL, and NOAA Great Lakes Aquatic Nonindigenous Species Information System, Ann Arbor, MI. https://nas.er.usgs.gov/queries/greatlakes/FactSheet.aspx?Species_ID=3610&Potential=Y&Type=2&HUCNumber=,%20Revision%20Date:%209/15/2021 (revision 9/15/2021, access February 27, 2023). [Google Scholar]
- Băcescu M. 1949. Données sur la faune carcinologiquede la Mer Noire de long de la côte Bulgare. Travaux Station Biol Maritime 4: 1–24. [Google Scholar]
- Băcescu M, Dumitrescu H. 1958. Les lagunes en formation aux embouchures du Danube et leur importance pour les poissons migrateurs. Verh Intern Ver Limnol 13: 699–709. [Google Scholar]
- Besacier-Monbertrand AL, Paillex A, Castella E. 2014. Short-term impacts of lateral hydrological connectivity restoration on aquatic macroinvertebrates. River Res Applic 30: 557–570. [CrossRef] [Google Scholar]
- Bij de Vaate A. 2003. Degradation and recovery of the freshwater fauna in the lower sections of the rivers Rhine and Meuse. Ph.D. Thesis, University of Wageningen, Wageningen. [Google Scholar]
- Bij de Vaate A, Jazdzewski K, Ketelaars HAM, Gollasch S, van der Velde G. 2002. Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Can J Fish Aquat Sci 59: 1159–1174. [CrossRef] [Google Scholar]
- Bij de Vaate A, Klink A, Paalvast P. 2007. Macrozoobenthos in the Lower Seine: a survey from the perspective of the European Water Framework Directive. Ecoconsult report: 200703. [Google Scholar]
- Cuthbert RN, Pattison Z, Taylor NG, Verbrugge L, Diagne C, Ahmed DA, Leroy B, Angulo E, Briski E, Capinha C, Catford J, Dalu T, Essl F, Gozlan RE, Haubrock PJ, Kourantidou M, Kramer AM, Renault D, Wasserman RJ, Courchamp F. 2021. Global economic costs of aquatic invasive alien species. Sci Total Environ 775: 145238. [CrossRef] [PubMed] [Google Scholar]
- de Carle DB, Gajda Ł, Bielecki A, Cios S, Cichocka JM, Golden HE, Gryska AD, Sokolov S, Borisowna Shedko M, Knudsen R,Utevsky S, Świątek P, Tessler, M. 2022. Recent evolution of ancient Arctic leech relatives: systematics of Acanthobdellida. Zool J Linnean Soc 196: 149–168. [CrossRef] [Google Scholar]
- Devin S, Akopian M, Fruget JF, DiMichelle A, Beisel JN. 2006. Premiéres observations écologiques dans les hydrosystémes Français Polychéte d'eau douce Hypania invalida introduit en Europe occidentale. Vie et Milieu − Life Environ 56: 1–8. [Google Scholar]
- Dobriyal P, Badola R, Tuboi CH, Hussain SA. 2017. A review of methods for monitoring stream flow for sustainable water resource management. Appl Water Sci 7: 2617–2628. [CrossRef] [Google Scholar]
- Downing JA, Cole JJ, Duarte CM, Middelburg JJ, Melack JM, Prairie YT, Kortelainen P, Striegl RG, McDowell WH, Tranvik LJ. 2012. Global abundance and size distribution of streams and rivers. Inland Waters 2: 229–236. [CrossRef] [Google Scholar]
- Dumnicka E. 2016. Alien Naididae species (Annelida: Clitellata) and their role in aquatic habitats in Poland. Biologia 71: 16–23. [Google Scholar]
- Dzyuban NA, Slobodchikov NB. 1980. Hypania invalida in the Volga River reservoirs Russian SFSR, USSR and hydrobiological monitoring. Hydrobiol Zh 16: 56–59. [Google Scholar]
- Eggers TO, Anlauf A. 2008. Hypania invalida (Grube, 1860) (Polychaeta: Ampharetidae) in der Mittleren Elbe. Lauterbornia 62: 11–13. [Google Scholar]
- Ferencz M. 1969. Occurence of Hypania invalida (GRUBE) in the Tisza (Annelida, Polychaeta). Tiscia (Szeged) 5: 69–71. [Google Scholar]
- Filinova E, Malinina YA, Shlyakhtin G. 2008. Bioinvasions in macrozoobenthos of the Volgograd Reservoir. Russ J Ecol 39: 193–197. [CrossRef] [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3: 294–299. [PubMed] [Google Scholar]
- Gallardo B, Aldridge DC. 2013. Review of the ecological impact and invasion potential of Ponto Caspian invaders in Great Britain. Cambridge Environmental Consulting, 120 pp. [Google Scholar]
- Gauch HG. 1982. Noise reduction by eigenvector ordinations. Ecology 63: 1643–1649. [CrossRef] [Google Scholar]
- Gherardi F, Gollasch S, Minchin D, Olenin S, Panov VE. 2009. Alien invertebrates and fish in European inland waters. In: Handbook of Alien Species in Europe. Springer Science + Business Media B.V. Dordrecht, The Netherlands, pp. 81–92. [CrossRef] [Google Scholar]
- Glasby CJ, Timm T. 2008. Global diversity of polychaetes (Polychaeta; Annelida) in freshwater. Hydrobiologia 595: 107–115. [CrossRef] [Google Scholar]
- Grabowski M, Bacela K, Konopacka A. 2007. How to be an invasive gammarid (Amphipoda: Gammaroidea) − comparison of life history traits. Hydrobiologia 590: 75–84. [CrossRef] [Google Scholar]
- Gruia L, Manoleli D. 1974. Considerations sur le role des algues dans la norriture des Polychetes reliqes ponto − caspiens du Danube. Trav Mus Nat 'Grigore Antipa' 15: 23–30. [Google Scholar]
- Hermanowicz W, Dojlido J, Dozanska W, Koziorowski B, Zerbe J. 1999. Fizyczno-chemiczne badania wody i ścieków. Arkady: Warszawa. [Google Scholar]
- Institute of Meteorology and Water Management - National Research Institute (IMWM‐NRI). (2020). Data of 2017–2020. [Google Scholar]
- Ketelaars HAM. 2004. Range extensions of Ponto-Caspian aquatic invertebrates in Continental Europe. In Dumont H, Shiganova TA, Niermann U, eds. Aquatic Invasions in the Black, Caspian, and Mediterranean Seas. Nato Science Series: IV: Earth and Environmental Sciences, Vol 35. Springer, Dordrecht. https://doi. org/10. 1007/1-4020-2152-6_13 [Google Scholar]
- Klink A, Bij de Vaate A. 1996. Hypania invalida (Grube, 1860) (Polychaeta: Ampharetidae) in the lower Rhine-new to the Dutch fauna. Lauterbornia 25: 57–60. [Google Scholar]
- Kozerska M. 2016. Inland waterway transport in Poland—the current state and prospects for development. Sci J Marit Univ Szczec 47: 136–140. [Google Scholar]
- Lvova AA, Paliy AV, Sokolova NY. 1996. Ponto-Caspian immigrants in the Moscow River within Moscow City. Zool Zh 75: 1273–1274. [Google Scholar]
- Manoleli D. 1975. On the distribution, biology and origin of Polychaeta from the Danube and the Danube Delta. Trav du Mus Natl Hist Nat Grigore Antipa 16: 24–33. [Google Scholar]
- Maruszczak M. 2019. The current state of inland navigation in Poland and its future development under European Union transport policy. Sci J Marit Univ Szczec 132: 47–55. [Google Scholar]
- Matysik M. 2019. Hazards to the quality of surface water from discharge of saline mining waters in the upper Oder River Basin. AIP Conf Proc 2186: 120007. [CrossRef] [Google Scholar]
- Mordukhai-Boltovskoi PD. 1964. Caspian fauna beyond the Caspian Sea. Int Rev gesamten Hydrobiol Hydrogr 49: 139–176. [CrossRef] [Google Scholar]
- Müller R, Hendrich L, Klima M, Koop JHE. 2006. Das Makrozoobenthos des Oder-Spree- der Fürstenwälder Spree in Brandenburg. Lauterbornia 56: 141–154. [Google Scholar]
- Müller R, Wolter C, Peschel T. 2018. Neobiota in Berliner Gewässern im Jahr 2018-Wirbellose Tiere, Fische und Wasserpflanzen. Bericht im Auftrag der Senatsverwaltung für Umwelt, Verkehr und Klimaschutz, Abteilung Integrativer Umweltschutz, Referat Wasserwirtschaft, 143 pp. [Google Scholar]
- Myslinska E. 2001. Organic and laboratory land testing methods. Panstwowe Wydawnictwo Naukowe. [Google Scholar]
- Norf H, Kniggendorf LG, Fischer A, Arndt H, Kureck A. 2010. Sexual and reproductive traits of Hypania invalida (Polychaeta, Ampharetidae): a remarkable invasive species in Central European waterways. Freshw Biol 55: 2510–2520. [CrossRef] [Google Scholar]
- Olden JD, Chen K, García-Berthou E, King AJ, South J, Vitule JR. 2021. Invasive species in streams and Rivers. Encyclopedia of Inland Waters. Elsevier. [Google Scholar]
- Olkowska E, Kudlak B, Tsakovski S, Ruman R, Simeonov V, Polkowska Z. 2014. Assessment of the water quality of Kłodnica River catchment using self‐organizing maps. Sci Total Environ 476–477: 477–484. [Google Scholar]
- Pabis K, Krodkiewska M, Cebulska K. 2017. Alien freshwater polychaetes Hypania invalida (Grube 1860) and Laonome calida Capa 2007 in the Upper Odra River (Baltic Sea catchment area). Knowl Manag Aquat Ecosyst 418: 1–3. [Google Scholar]
- Pavel AB, Menabit S, Pop IC, Stanescu I, Naliana L. 2021. The spatio-temporal distribution of the Ponto-Caspian polychaete in the Lower Sector of the Danube River and in Danube Delta. Glob Ecol Conserv 28: e01623. [Google Scholar]
- Popescu-Marinescu V. 1992. Les populations d‘Hypania invalida (Grube) dans la region Portile de Fier, avant et après la création du lac d‘accumulation. Rev Roumaine Biol Sér Biol Anim 37: 131–139. [Google Scholar]
- Pyšek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, Carlton JT, Dawson W, Essl F, Foxcroft LC, Genovesi P, Jeschke JM, Kühn I, Liebhold AM, Mandrak NE, Meyerson LA, Pauchard A, Pergl J, Roy HE, Seebens H, van Kleunen M, Vilà M, Wingfield MJ, Richardson DM. 2020. Scientists' warning on invasive alien species. Biol Rev 95: 1511–1534. [CrossRef] [PubMed] [Google Scholar]
- Schmidt WD, Kaiser I, Schuller I. 1998. Zwei Neuankömmlinge aus der Donau − Hypania invalida (Polychaeta) und Jaera istri (Isopoda) − haben den ganzen Main besiedelt. Lauterbornia 33: 121–123. [Google Scholar]
- Semenchenko V, Son M, Novitski R, Kvach Y, Panov V. 2016. Checklist of non-native benthic macroinvertebrates and fish in the Dnieper River basin. BioInvasions Rec 5: 185–187. [CrossRef] [Google Scholar]
- Shcherbina GK. 2001. Autoacclimatization of the Ponto-Caspian polychaete Hypania invalida from the Upper Volga River basin. Zool Zhurnal 80: 278–284. [Google Scholar]
- Shcherbina GK. 2012. Role of bottom macrozoobenthic invertebrates in feeding of fishes-benthophages in Volga reservoirs, in Mater. Vseross. konf. “Bassein Volgi v XXI veke: struktura i funktsionirovanie ekosistem vodokhranilishch” (Proc. All-Russ. Conf. “The Volga Basin in 21st Century: Structure and Functions of Reservoir Ecosystems”), Izhevsk: Izd. Permyakov S.A., pp. 366–368. [Google Scholar]
- Stiller J, Tilic E, Rousset V, Pleijel F, Rouse GW. 2020. Spaghetti to a tree: a robust phylogeny for Terebelliformia (Annelida) based on transcriptomes, molecular and morphological data. Biology 9: 73. [CrossRef] [PubMed] [Google Scholar]
- Straka M, Špaček J, Pařil P. 2015. First record of the invasive polychaete Hypania invalida (Grube, 1960) in the Czech Republic. BioInvasions Rec 15: 87–90. [CrossRef] [Google Scholar]
- Surugiu V. 2005. The use of polychaetes as indicators of eutrophication and organic enrichment of coastal waters: A study case − Romanian Black Sea coast. Analele Ştiinţifice ale Universităţii “Al. I. Cuza’’ Iaşi, s. Biologie animală 51: 55–62. [Google Scholar]
- Szító A. 1996. A Tisza üledéklakó életközösségének változása az első adatoktól napjainkig (The changes in macrozoobenthos of the River Tisza from the first data to nowadays). Hidrológiai Közlöny 1: 19–37. [Google Scholar]
- Šmilauer P, Lepš J. 2014. Multivariate Analysis of Ecological Data Using CANOCO 5. Cambridge University Press. [Google Scholar]
- Tischikov GM, Tischikov IG. 2005. Taxonomic composition of macrozoobenthic communities in the Zapadnyi Bug and Narev rivers’ basins, in Proceedings of the 7th National scientific conference ‘Use of the rivers Narev and Bug in the context of sustainable development’. Warsaw-Debe, pp. 175–191 [in Russian with English summary] [Google Scholar]
- Tittizer T, Schöll F, Banning M, Haybach A, Schleuter M. 2000. Aquatische Neozoen im Makrozoobenthos der Binnenwasserstarssen Deutschlands. Lauterbornia 39: 1–72. [Google Scholar]
- Vanden Bossche J. 2002. First records and fast spread of five new (1995–2000) alien species in the River Meuse in Belgium: Hypania invalida, Corbicula fluminea, Hemimysis anomala, Dikerogammarus villosus and Crangonyx pseudogracilis. Peeters M,Van Goethem J. “Actes du symposium” Faune belge et espèces exotiques. Bulletin van het Koninklijk Belgisch Instituut voor Natuurwetenschappen-Biologie, pp. 73–78. [Google Scholar]
- Vanden Bossche JP, Chérot F, Delooz E, Grisez F, Josens G. 2001. First record of the Pontocaspian invader Hypania invalida (Grube, 1860) (Polychaeta: Ampharetidae) in the River Meuse (Belgium). Belg J Zool 131: 183–185. [Google Scholar]
- Vučković N, Pozojević I, Urbanič G, Mihaljević Z. 2021. New evidence supporting upstream pathways of Hypania invalida (Grube, 1860) invasion. BioInvasions Rec 10: 589–597. [CrossRef] [Google Scholar]
- Wozniczka A, Gromisz S., Wolnomiejski N. 2011. Hypania invalida (Grube, 1960), a polychaete species new for the southern Baltic estuarine area: the Szczecin Lagoon and the River Odra mouth. Aquat Invasions 6: 39–46. [CrossRef] [Google Scholar]
- Yakovlev VA, Yakovleva AV. 2010. Polychaeta Hypania invalida (Polychaeta: Ampharetidae) in the Kuybyshev Reservoir: distribution and size and weight parameters. Russ J Biol Invasions 1: 153–159. [CrossRef] [Google Scholar]
- Zorić K, Jakovčev-Todorović D, Dikanović V, Vasiljević B, Tomović J, Atanacković A, Simić V, Paunović M. 2011. Distribution of the Ponto-Caspian polychaeta Hypania invalida (Grube, 1860) in inland waters of Serbia. Aquat Invasions 6: 33–38. [CrossRef] [Google Scholar]
Cite this article as: Krodkiewska M, Cebulska K, Gajda Ł, Świątek P. 2024. Distribution of the invasive polychaete Hypania invalida (Grube, 1860) against the background of the benthic fauna in the upper Oder River catchment (Poland). Knowl. Manag. Aquat. Ecosyst., 425. 2
All Tables
Abundance of macroinvertebrates (per m2) at the sites where H. invalida was found.
Relative abundance (%) of alien versus native macroinvertebrates in benthic fauna at the sites where H. invalida occurred.
Summary of canonical correspondence analysis (CCA) performed on macroinvertebrate data and environmental variables determined by forward selection.
All Figures
 |
Fig. 1 The first records of H. invalida in European rivers outside the area of natural occurrence based on reference data. Abbreviation: sof references: 1Popescu-Marinescu (1992), 2Semenchenko et al. (2016), 3Ketelaars (2004), 4Dzyuban and Slobodchikov (1980), 5Ferencz (1969), 6Zorić et al. (2011), 7Lvova et al., (1996), 8Klink and Bij de Vaate (1996), 9Tittizer et al. (2000), 10Tischikov and Tischikov (2005), 11Vanden Bossche et al. (2001), 12Devin et al. (2006), 13Eggers and Anlauf (2008), 14Gallardo and Aldridge (2013), 15Wozniczka et al. (2011), 16Müller et al. (2018), 17Vučković et al. (2021). |
| In the text | |
 |
Fig. 2 Area of the study. Abbreviations of study sites: the Oder River (O1-O3), the Gliwice Canal (KG1-KG11), the Kłodnica Canal (KK), the Kłodnica River (K1-K17), tributaries of the Kłodnica River (M-Młynówka Stream, Jo-Jordan Stream, PT-Toszecki Stream, D-Drama River, B-Bytomka River, C-Czerniawka River, PB-Bielszowicki Stream, P-Promna Stream, Ja-Jamna Stream, S-Ślepiotka Stream). |
| In the text | |
 |
Fig. 3 Ordination diagram based on the canonical correspondence analysis of the taxa abundance data and the best explanatory variables. Abbreviations: alien species Ch.cur-Ch.curvispinum, C.flu-C.fluminea, D.hae-D.haemobaphes, D.pol-D.polymorpha, D.vill-D.villosus, G.tig-G.tigrinus, H.inv-H.invalida, L.xep-L.xeprovala, P.acu-P.acuta, P.bar-P.barbatus, P.bav-P.bavaricus, P.alb-P.albicola, P.ant-P.antipodarum, other macroinvertebrate taxa Asel-Asellidea, Baet-Baetidae, Bith-Bithyniidae, Caen-Caenidae, Cera-Ceratopogonidae, Chiro-Chironimidae, Coen-Coenagrionidae, Coro-Corophidae, Dyti-Dytiscidae, Ecno-Ecnomidae, Ench-Enchytraeidae, Erpo-Erpobdellidae, Gloss-Glossiphoniidae, Hali-Haliplidae, Hydr-Hydropsychidae, Lymn-Lymnaeidae, Meso-Mesoveliidae, Naid-Naididae, Psycho-Psychodidae, Simu-Simuliidae, Spha-Sphaeriidae, Veli-Veliidae, Vivi-Viviparidae, environmental variables Mod-hydromorphologically modified river stretch, Nat-natural river stretch |
| In the text | |
 |
Fig. 4 (A) Sediment with tubes of H. invalida from the upper Oder River; (B) Hypania invalida. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


