| Issue |
Knowl. Manag. Aquat. Ecosyst.
Number 418, 2017
|
|
|---|---|---|
| Article Number | 58 | |
| Number of page(s) | 11 | |
| DOI | https://doi.org/10.1051/kmae/2017044 | |
| Published online | 27 November 2017 | |
Research Paper
The potential habitats of two submerged macrophytes, Myriophyllum spicatum and Hydrilla verticillata in the river ecosystems, South Korea
Les habitats potentiels de deux macrophytes submergés, Myriophyllum spicatum et Hydrilla verticillata dans les écosystèmes fluviaux, Corée du Sud
1
School of Biological Sciences, Seoul National University,
Seoul
08826, Republic of Korea
2
Department of Biological Sciences, Inha University,
Incheon
22212, Republic of Korea
* Corresponding author: ejlee@snu.ac.kr
We examined the environmental factors that characterize the habitats of two submerged macrophytes, Myriophyllum spicatum and Hydrilla verticillata, in South Korea using generalized additive models (GAMs). We performed a vegetation survey and measured water depth and water velocity from May to September, 2014–2015. Averaged water quality data from the Ministry of Environment's national water quality measurement network from January 2012 to October 2015 were used for modeling. Potential habitats of M. spicatum were linked with chlorophyll a, nitrate nitrogen, suspended solids, water temperature, water depth, and water velocity (deviance explained = 28.7%, accuracy = 74%). In the case of H. verticillata, electrical conductivity and suspended solids were key habitat factors (deviance explained = 23.4%, accuracy = 75%). Model results were highly consistent with observations and field data. Model performances were evaluated by the accuracy rate, the area under the receiver operating characteristic curve, the kappa value, and field verification, and were in generally good agreement. Few studies have evaluated the developed models using the independent field data. By understanding the environmental factors that characterize the habitats for submerged macrophytes, our results contribute to the development of conservation and management strategies for river ecosystems.
Résumé
Nous avons examiné les facteurs environnementaux qui caractérisent les habitats de deux macrophytes submergés, Myriophyllum spicatum et Hydrilla verticillata, en Corée du Sud à l'aide de modèles additifs généralisés (GAMs). Nous avons effectué un relevé de la végétation et mesuré la profondeur et la vitesse de l'eau de mai à septembre 2014-2015. Les données moyennes sur la qualité de l'eau provenant du réseau national de mesure de la qualité de l'eau du ministère de l'Environnement pour la période de janvier 2012 à octobre 2015 ont été utilisées pour la modélisation. Les habitats potentiels de M. spicatum étaient liés à la chlorophylle a, au nitrate, aux solides en suspension, à la température de l'eau, à la profondeur et à la vitesse de l'eau (variance expliquée = 28,7 %, précision = 74 %). Dans le cas de H. verticillata, la conductivité électrique et les solides en suspension étaient des facteurs clés de l'habitat (variance expliquée = 23,4 %, précision = 75 %). Les résultats des modèles étaient très cohérents avec les observations et les données de terrain. Les performances du modèle ont été évaluées par le taux de précision, la surface sous la courbe caractéristique de fonctionnement du récepteur, la valeur kappa et la vérification sur le terrain, et elles ont été généralement bien concordantes. Peu d'études ont évalué des modèles élaborés à l'aide des données indépendantes sur le terrain. En comprenant les facteurs environnementaux qui caractérisent les habitats des macrophytes submergées, nos résultats contribuent à l'élaboration de stratégies de conservation et de gestion des écosystèmes fluviaux.
Key words: Eurasian watermilfoil / Hydrilla / water condition / habitat suitability / generalized additive model
Mots clés : myriophylle eurasienne / Hydrilla / qualité de l'eau / convenance de l'habitat / modèle additif généralisé
© D. Son et al., Published by EDP Sciences 2017
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (http://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (http://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
1 Introduction
Submerged macrophytes play an important role as a producer in food webs, shelter and forage for other organisms, and as a water quality indicator (Nieder et al., 2004). In addition, submerged macrophytes produce oxygen in stagnant regions and prolong the hydrologic retention time for the removal of particulate nutrients (Nepf et al., 2007). Despite the importance of submerged macrophytes, the formation of dense monotypic stands has adverse effects on the diversity and richness of invertebrates and fish (Buchan and Padilla, 2000). Dense beds of submerged macrophytes can produce organic materials from actively growing or senescing macrophytes and cause eutrophication of the water column (Chambers et al., 1999). Moreover, their proliferation can impede water flow, clog inlets of reservoirs, and interfere with recreational activities (Kenneth, 1996).
The spatial distribution of organisms is related to species dispersal and survival at a regional scale. In addition, abiotic conditions (environmental constraints) and biotic interactions (e.g., competition and herbivory) influence species distributions at a local scale (Austin, 2002; Bučas and Bergström, 2013; Chappuis et al., 2014). The occurrence and abundance of submerged macrophytes are influenced by chemical and physical factors, such as water quality, light availability (Dennison et al., 1993), water transparency, water depth (Canfield et al., 1985), channel slope, channel dimensions (O'Hare et al., 2011), and hydrological regime (Franklin et al., 2008). Understanding how diverse environmental factors affect the habitats of submerged macrophytes is important for flow control, sediment transport (Järvelä, 2005), and assessments of the ecological condition of rivers (Clayton and Edwards, 2006).
A variety of statistical approaches such as generalized linear models and generalized additive models (GAMs) are important tools for predicting the likely occurrence or distribution of a species (Pearce and Ferrier, 2000; Austin, 2002). In particular, GAMs are used extensively in habitat suitability modeling and identification of the optimal environmental conditions for a given species; data are fitted using a semi-parametric model to predict non-linear responses to the exploratory variables (Elith et al., 2006; Drexler and Ainsworth, 2013; Li and Wang, 2013). GAMs not only have a strong statistical foundation, but can be used to realistically model ecological relationships (Yee and Mitchell, 1991; Sanchez et al., 2008). When the relationship between a species distribution and environmental variables is complex, GAMs are practical and perform as well or better than other types of predictive models (Drexler and Ainsworth, 2013; Li and Wang, 2013). GAMs have been used to examine potential seagrass habitats (Lathrop et al., 2001; Downie et al., 2013), fish production and distributions (Borchers et al., 1997; Buisson and Blanc, 2008; Murase et al., 2009; Solanki et al., 2016), and terrestrial plant distributions (Yee and Mitchell, 1991; Austin and Meyers, 1996; Thuiller et al., 2005), but few studies have used GAMs to examine submerged macrophytes in river ecosystems.
Recently, river ecosystems in South Korea have experienced channel dredging, channelization, and dam construction for flood control during rainfall periods and to secure water resources during drought periods (Woo, 2010). In particular, the “Four Major Rivers Project” (2009–2012) involved the construction of 16 weirs and three dams in the Han, Geum, Nakdong, and Yeongsan Rivers (Lah et al., 2015). This national project aimed to secure water resources, reduce flooding, improve water quality, and create multipurpose public spaces for local residents (Jun and Kim, 2011). Despite substantial controversy surrounding the effectiveness of this project (Normile, 2010), it drastically changed the natural riverine habitats and enabled the artificial manipulation of the water level and the regulation of water flow (Lee et al., 2014). The modified slow velocity in regulated streams may increase the abundance of macrophytes (Bunn and Arthington, 2002) and devastate habitats for organisms adapted to the natural discharge regime (Dynesius and Nilsson, 1994).
Alterations to hydrological regimes affect the structure and function of aquatic ecosystems, resulting in changes in the spatial distributions of submerged macrophytes (Tian et al., 2015). Submerged macrophytes in the river ecosystems of South Korea were found to change from slow flowing streams to large rivers after weir construction. Myriophyllum spicatum L. (Eurasian watermilfoil) and Hydrilla verticillata (L. f.) Royle (Hydrilla) are native species in South Korea; however, they are fast growing and the most abundant macrophytes in Korean rivers. They are invasive species and strong competitors in Europe, the United States, and South America owing to their rapid and dense growth (Van et al., 1999; Gassmann et al., 2006; Beck et al., 2008). These two species are cosmopolitan angiosperms with extensive worldwide ranges (Zhou et al., 2016) and overrun various habitats, from lentic to lotic systems, and in turn affect flow velocity and nutrient cycling in the water column (Sousa, 2011).
Accordingly, it is necessary to understand the current distributions as well as predict suitable habitats of submerged macrophytes with high invasive potentials for river management and conservation planning. Few studies have predicted the distributions of submerged macrophytes in the rivers using GAMs (Ahmadi-Nedushan et al., 2006; Camporeale and Ridolfi, 2006). Moreover, the developed GAMs have rarely been validated via independent field data (Guisan et al., 2002). The aims of the study were to examine under what environmental conditions M. spicatum and H. verticillata are likely to occur in and to predict and validate their suitable habitats. Our specific objectives were (1) to characterize the relationship between environmental variables and the occurrence of two submerged macrophytes (M. spicatum and H. verticillata) using GAMs, (2) to predict the suitable habitats for these two submerged macrophytes, and (3) to evaluate GAMs by applying the independent field data.
2 Materials and methods
2.1 Study sites and data collection
The study sites were located in the central and southern parts of the Korean Peninsula (33°–39° N, 124°–130° E) which has a temperate climate. The mean annual precipitation is approximately 1300 mm, and the mean annual temperature is 10–14 °C (Korea Meteorological Aministration, http://www.kma.go.kr/weather/climate/average_normal.jsp). Two-thirds of the annual precipitation occurs in the summer (June–August). The winter season (December–February) tends to be dry and cold, reflecting a typical continental weather pattern (Shin and Chung, 2011).
Surveyed streams and rivers converge into five major rivers: Han River, Geum River, Nakdong River, Yeongsan River, and Seomjin River (Fig. 1). For the vegetation survey, 197 sites in the rivers and tributaries were randomly selected, including 71 sites in the Han River, 43 sites in the Geum River, 46 sites in the Nakdong River, 27 sites in the Yeongsan River, and 10 sites in the Seomjin River. Sites were assigned to water quality monitoring towers operated by the Ministry of Environment. The presence and absence of submerged macrophytes were recorded using a belt transect (50 m × 2 m) parallel to the direction of water flow from May to September in 2014–2015 (Dawson et al., 1999; Riis et al., 2001) and from June to July in 2016 for field verification.The belt transect was spaced 1 m from the edge of the river. Submerged macrophytes are rarely observed at depths exceeding 2.5 m in rivers and streams (Riis et al., 2001; Angradi et al., 2013); examinations were performed by wading or in a boat with a rake for confirmation (Park et al., 2013).
Water quality data were acquired from the Ministry of Environment's national water quality measurement network (http://water.nier.go.kr) from January 2012 to October 2015. Averaged water quality data calculated from monthly measurements were used for modeling. We chose 14 water environmental variables that had a postulated relationship with the ecological requirements of submerged macrophytes (Gallego et al., 2015; Klippel et al., 2016), namely, water temperature, pH, biochemical oxygen demand (BOD), chemical oxygen demand (COD), suspended solids, total nitrogen, ammonium nitrogen, nitrate nitrogen, total phosphorus, total organic carbon, electrical conductivity, total dissolved nitrogen, total dissolved phosphorus, and chlorophyll a. In each transect, water depth (meter stick) and water flow (Flowatch; JDC Electronic SA, Yverdon-les-Bains, Switzerland) were measured three times where vegetation was most abundant, and the values were averaged. If submerged macrophytes were lacking, water depth and water flow were measured in triplicate at the midpoint of the transect. We recorded channel structure and confluence points according to in situ survey and stream order data obtained from the Water Resources Management Information System (the Ministry of Land, Infrastructure and Transport, www.wamis.go.kr).
 |
Fig. 1 Studied streams in South Korea. The streams were roughly classified into five rivers: Han River, Geum River, Nakdong River, Yeongsan River, and Seomjin River. |
2.2 Model building
All statistical analyses were performed in R (R Development Core Team, 2016). Prior to the statistical analysis, all explanatory variables were log10-transformed to improve normality (Chappuis et al., 2014). Pearson correlation analyses were performed to detect high multicollinearity (Zhao et al., 2014; Wedding and Yoklavich, 2015) and the variables that have correlation coefficient greater than 0.75 with another variable were eliminated (Kuhn and Johnson, 2013) (Tab. S1). Total nitrogen, BOD, COD, ammonium nitrogen, total phosphorus, total organic carbon, and total dissolved nitrogen were excluded because they were highly correlated with suspended solids, nitrate nitrogen, and total dissolved phosphorus. Multivariate statistical methods, such as principal component analysis (PCA), have been recommended to determine the main environmental factors before inclusion in models (Shmueli, 2010; Zhao et al., 2014). Six significant elements of water environmental factors determined via PCA were selected and two elements from in situ measurements (water depth and water velocity) were added to the GAMs (Tab. S2). Finally, eight environmental descriptors were included in GAMs: chlorophyll a, electrical conductivity, nitrate nitrogen, suspended solids, total dissolved phosphorous, water temperature, water depth, and water velocity. All variables describing the physical and chemical properties of water included in the GAMs are described in Table 1.
We used GAMs to approximate the probability of taxon presence with respect to the predictors. A binomial distribution was specified (presence = 1 and absence = 0) with a logit link function relating the dependent variables to the predictors. Such approach allows modeling presence–absence data at survey sites, thereby enabling the probability of species occurrence to be predicted from independent data at unsurveyed sites (Pearce and Ferrier, 2000). The descriptor variables were modeled as cubic splines, with 4° of smoothing (Lehmann, 1998; Wood, 2000), using the “mgcv” library for GAMs (Wood, 2006). The most parsimonious model for each species with the fewest variables was chosen using a stepwise selection procedure (Buisson and Blanc, 2008). To determine the best fit model, Akaike's information criterion (AIC) was used as a goodness-of-fit statistic (Zuur and Pierce, 2004; Buisson and Blanc, 2008; Sanchez et al., 2008). Models with a smaller AIC were able to explain the residual deviance better than those with a larger AIC. The best model accounted for the most variation in the data using the fewest predictors (Burnham and Anderson, 1998).
Mean, standard error (SE), minimum (Min), and maximum (Max) values for physical and chemical properties of water at 197 sites. Chlorophyll a, electrical conductivity, total dissolved phosphorus, nitrate nitrogen, and suspended solids are presented as mean values of monthly estimates from 2012 to 2015, water temperature is presented as the mean from May to October, and water depth and velocity data were obtained at the sampling date.
2.3 Validation of the predictive performance of models
To obtain an unbiased estimation of model performance, it is best to apply independent data that have not been used for model development (Pearce and Ferrier, 2000). However, if independent data are not available, a k-fold cross-validation may be used to assess model accuracy (Pearce and Ferrier, 2000; Zimmermann et al., 2007). We applied k-fold cross-validation (with k = 10); the data were randomly split into two datasets: 90% (k − 1 subsamples) was used as a training dataset to build a model and the remaining 10% (one subsample) was used as a testing dataset for validation. This procedure was repeated ten times to calculate the probabilities of occurrence, which were transformed into binary records (presence/absence) using a threshold probability (Sing et al., 2005; Araújo and Luoto, 2007). To generate a confusion matrix, the Youden index was chosen as the threshold probability, which was the maximum difference between sensitivity (the probability of correct classification as positive) and specificity (the probability of correct classification as negative) (Jiménez-Valverde and Lobo, 2007; Freeman and Moisen, 2008), using the “SDMTools” library for model accuracy (VanDerWal et al., 2014). Coordinates of observations and predictions based on the confusion matrix were projected to World Geodetic System 84 (WGS84) using QGIS (QGIS Development Team, 2016).
Model accuracy was assessed by two measures: Cohen's kappa and the area under the receiver operating characteristic (ROC) curve (AUC) (Zimmermann et al., 2007). Cohen's kappa is the most common method for determining the accuracy of presence–absence predictions based on a selected threshold probability, regardless of variation in prevalence (Segurado and Araujo, 2004; Allouche et al., 2006). Landis and Koch (1977) suggested the following interpretation of kappa values: excellent agreement, >0.75; good agreement, 0.40–0.75; and poor agreement, <0.40. Another method for assessing the accuracy of models uses the AUC value as a threshold-independent criterion (Fielding and Bell, 1997). To construct ROC curves, all possible thresholds were used to classify the scores into confusion matrices, and the sensitivity and specificity were estimated for each matrix (Allouche et al., 2006). According to Swets (1988), AUC values were interpreted as follows: excellent, >0.90; good, 0.80–0.90; fair, 0.70–0.80; poor, 0.60–0.70; fail, 0.50–0.60. Moreover, in situ observations of the 41 sites were randomly performed to evaluate the accuracy of the model for field verification.
3 Results
3.1 GAM response curves
We defined the ranges of environmental factors with respect to species occurrences. The models explained 28.7% and 23.4% of the observed variation in selected variables for M. spicatum and H. verticillata, respectively (Tab. 2). For M. spicatum, we found that chlorophyll a, nitrate nitrogen, suspended solids, water temperature, water depth, and water velocity were significant variables in the GAM. Based on the response curves, the probability of M. spicatum presence increased as nitrate nitrogen increased, and decreased as water temperature and suspended solids increased (Fig. 2A). The response curves for chlorophyll a indicated a sharp increase in the predicted presence of M. spicatum from 0 to ∼20 mg/m3 and a decline at higher concentrations. The presence probabilities of M. spicatum for water velocity decreased for values of up to ∼0.4 m/s and then increased in relatively rapid flows. Myriophyllum spicatum was distributed in a wide range of water depths up to 1 m and decreased in deeper water. Electrical conductivity and suspended solids were important parameters determining the H. verticillata distribution (Tab. 2). Hydrilla verticillata presence was negatively related to electrical conductivity and suspended solid concentrations (Fig. 2B). We also observed differences in the predicted potential habitats between the two species (Fig. 3). Myriophyllum spicatum was widely distributed in the Han River and Nakdong River, and its predicted distribution was similar to its current distribution. Hydrilla verticillata was abundant everywhere, whereas its distribution was predicted to be decreased in Nakdong River and increased in Yeongsan River.
Selected environmental variables and deviance explained in GAMs for Myriophyllum spicatum and Hydrilla verticillata.
 |
Fig. 2 Response curves of (A) Myriophyllum spicatum and (B) Hydrilla verticillata for environmental gradients in GAMs. The vertical axes represent the probabilities of occurrence, and shaded bands show the 95% confidence interval. Rug plots on the x-axis show data points. Chla, chlorophyll a; EC, electrical conductivity; NO3N, nitrate nitrogen; SS, suspended solids; WT, water temperature; WD, water depth; WV, water velocity. |
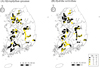 |
Fig. 3 Predicted and observed habitat suitability of (A) Myriophyllum spicatum and (B) Hydrilla verticillata based on GAMs. Observed occurrence (Ob.) is overlaid with the predicted occurrence (Pr.). The outer circle indicates the observed distribution, and the inner circle represents the predicted distribution. Yellow indicates presence (1) and black indicates absence (0). The same color for the outer and inner circles shows that observations and predictions coincide. A black outer circle with a yellow inner circle indicates a false positive, and a yellow outer with a black inner circle indicates a false negative. |
3.2 Model validation and field verification
To assess model performances for each species, we compared predicted potential habitats with observed habitats using the confusion matrix (Tab. 3). We observed accuracy rates of 0.74 for M. spicatum and 0.75 for H. verticillata. For M. spicatum, we observed AUC = 0.84 and kappa = 0.46 when we applied a threshold of 0.536. For H. verticillata, we observed AUC = 0.79 and kappa = 0.39 when the threshold was 0.492. Based on the field verification, we observed accuracy rates of 76% for the two species (Tab. 4). In addition, we established AUC values of 0.75 for M. spicatum and 0.82 for H. verticillata, and kappa values of 0.44 for M. spicatum and 0.25 for H. verticillata.
Comparison of predicted and observed distributions of Myriophyllum spicatum and Hydrilla verticillata during model building. Predicted values were obtained from the fitted probability of presence using the Youden index to apply a threshold probability: 0.536 for M. spicatum and 0.492 for H. verticillata.
Comparison of predicted and observed distributions of Myriophyllum spicatum and Hydrilla verticillata at the model confirmation stage. Predicted values were obtained from the fitted probability of presence using the Youden index to apply a threshold probability: 0.536 for M. spicatum and 0.492 for H. verticillata.
4 Discussion
In this study, we identified factors that characterize the suitable habitats of M. spicatum and H. verticillata using GAMs, namely, chlorophyll a, electrical conductivity, nitrate nitrogen, suspended solids, water temperature, water depth, and water velocity. GAMs are very useful for describing the complex relationships between response variables and environmental factors (Cheng and Gallinat, 2004); however, our models explained low proportions of deviance (Tab. 2). This can be explained by the patchy distribution of submerged macrophytes, which cannot be fully explained by the selected variables (Lehmann, 1998). Nevertheless, based on the model evaluation procedures, the response curves for each variable enable us to infer general trends and we expect to adapt models to other locations around the world.
4.1 GAM results and environmental factors
The abundance and distribution of submerged macrophytes in river ecosystems are related to water quality conditions (Nieder et al., 2004), water depth, and water velocity (Sousa, 2011). We found that variables associated with water environmental factors were important determinants of the distributions of M. spicatum and H. verticillata, especially chlorophyll a, electrical conductivity, nitrate nitrogen, suspended solids, water temperature, water depth, and water velocity. Gradients of these variables also determine suitable habitats for submerged macrophytes in previous studies (Dodkins et al., 2005; Lacoul and Freedman, 2006; Franklin et al., 2008).
Our results demonstrated that the occurrence of M. spicatum increased for low concentrations, and decreased for high concentrations of chlorophyll a. In addition, the probability of M. spicatum presence decreased as water temperature increased. A higher temperature within optimal ranges usually promotes a higher chlorophyll a concentration and productivity as well as a greater abundance of submerged macrophytes (Barko et al., 1986). However, competition for light between aquatic plants and phytoplankton may limit plant growth and even result in the disappearance of taxa (Rybicki and Landwehr, 2007; Bornette and Puijalon, 2011). Jones et al. (1983) also found that phytoplankton-created low water clarity inhibits M. spicatum growth. Furthermore, the adaptability of submerged macrophytes to low temperatures may play a role in interspecific competition because the optimal water temperature for submerged macrophytes is 28–32 °C (Barko et al., 1986).
The occurrence of H. verticillata was high when electrical conductivity was low in oligotrophic water (Fig. 2B). This agrees with the findings of Sousa et al. (2009)and Cook and Lüönd (1982) who found H. verticillata develops well in oligotrophic waters.Plant growth usually increases as the concentration of nutrients in water and sediment increases (Van et al., 1999; Yu et al., 2010; Sousa, 2011). Moreover, Kennedy et al. (2009) found that H. verticillata can thrive not only in eutrophic waters, but also in oligotrophic waters. According to Sousa (2011), however, eutrophic conditions may have negative effects on H. verticillata growth via the proliferation of plankton, which compete with submerged macrophytes for light and nutrients. Electrical conductivity, as a measure of the chemicals summary variable (Heegaard et al., 2001), may affect macrophyte composition and be unfavorable for submerged macrophytes that are sensitive to eutrophication (Thomaz et al., 2003; Lauridsen et al., 2015). The occurrence of M. spicatum was high when nitrate nitrogen was high in water (Fig. 2A). Nitrogen is a key element for aquatic plants, which use nitrate as a nitrogen source (Bornette and Puijalon, 2011) and M. spicatum was related to with higher nitrate availability (Ali and Soltan, 2006).
We detected abundant M. spicatum in water at depths of 0.7–1.0 m, and a decreased abundance in deeper water (Fig. 2A). This result concurs with a previous study; Nichols and Shaw (1986) reported that M. spicatum is typically found in water ranging from 1 to 4 m deep. Angradi et al. (2013) observed that the optimal depth for submerged macrophytes is ∼1.2 m and <1 m in turbid conditions. However, an increase in water depth causes a light deficiency for submerged macrophytes (Bornette and Puijalon, 2011), thereby decreasing the rate of photosynthesis. Shallow water bodies allow more light penetration, provided that the water is not turbid (Narumalani et al., 1997). Lower water clarity owing to sediments, turbidity, and nutrients can reduce the water depth and spatial distribution for growth and survival of submerged macrophytes (Dar et al., 2014; Patrick et al., 2014). Consequently, as depth increases or water clarity decreases, light availability for photosynthesis may diminish (Lacoul and Freedman, 2006).
Based on the velocity response curve, we observed a decrease in M. spicatum abundance for values of up to ∼0.4 m/s and an increase for higher values (Fig. 2A). On the other hand, M. spicatum has been found in the stream at water velocities of 2 m/s (Nichols and Shaw, 1986). A number of water velocity readings in this study were at 0 m/s, with an average of 0.1 m/s and a median of 0 m/s. The regulated rivers and streams in study sites were close to lentic conditions; accordingly, we could not precisely evaluate the velocity response of M. spicatum. However, in general, the biomass and richness of submerged macrophytes are higher at 0.3–0.4 m/s, and lower at higher velocities because of failure to establish and colonize (Lacoul and Freedman, 2006). Moderate flow can encourage submerged macrophyte growth by continuously washing photosynthetic tissues covered with epiphytic algae (Strand and Weisner, 1996; Lehmann, 1998).
4.2 Model validation and field verification
We did not develop GIS-based predictive maps to identify potential distribution for submerged macrophytes because it was difficult to construct a bathymetric map of all rivers and streams in South Korea. We present maps showing areas of agreement between observations and predictions. Although we did not generate spatial maps interpolated with predicted probabilities, we were able to detect areas with abundant submerged macrophytes in the five rivers and characterize the suitable habitats of them.
Correct and incorrect predictions in a confusion matrix indicate the strength of predictions (Peters et al., 2007). The misclassified sites in the predicted distribution according to GAMs were related to channel structure, rather than water quality. Most of these sites were confluence points, i.e., sites at which two channels met, each carrying independent influxes and sediment discharge (Benda et al., 2004). To examine false positive errors (commission error; observation = 0 and prediction = 1), we considered the characteristics of survey sites at confluence points that connected relatively larger tributaries to main water bodies. Confluences have been described as biodiversity hot spots with physical heterogeneity and habitat complexity owing to diverse physical, chemical, and biological attributes resulting from tributary streams (Kiffney et al., 2006; Rice et al., 2006). At these sites, we expected to observe submerged macrophytes; however, their distributions were discontinuous and bed sediment size and flow properties were unstable (Rice et al., 2006). Slow velocity conditions and the maintenance of stable substrates are necessary for macrophytes colonization (Riis and Biggs, 2003). False positive results imply that submerged macrophytes did not have an opportunity to establish to a suitable habitat (Buchan and Padilla, 2000).
We observed false negative errors (omission error; observation = 1 and prediction = 0) at study sites that were typically downstream of confluence points connecting relatively small tributaries and irrigation ditches with the potential for submerged macrophytes dispersal. Myriophyllum spicatum and H. verticillata are found in rivers, lakes, irrigation ditches, and other waterways (Netherland, 1997; Eiswerth et al., 2000). Their primary dispersal strategy is vegetative reproduction by fragmentation, moving through small waterways and then establishing at channel junctions. Vegetative reproduction by stem fragmentation is an efficient mechanism for dispersal, colonization, and overwintering (Sousa, 2011; Xie et al., 2013). The conditions downstream of small tributaries are sufficiently stable for submerged macrophytes inhabitation. Submerged macrophytes habitats are regulated by a variety of factors, varying within not only whole streams, but also smaller stream reaches (Riis et al., 2001).
We observed high accuracy rates for each species model (0.74 for M. spicatum and 0.75 for H. verticillata), but low proportions of variation explained by the models. We observed kappa values (which were dependent on a threshold) of 0.46 for M. spicatum and 0.39 for H. verticillata, indicating a fair model fit. In addition, we observed AUC values (independent of threshold values) of 0.84 for M. spicatum and 0.79 for H. verticillata, indicating satisfactory predictive ability. According to the AUC and kappa values, model performance was good for both species. Field verification to validate favorable potential habitats for M. spicatum and H. verticillata confirmed model performance, supporting their good prediction abilities, based on accuracy rates, AUC, and kappa values, except for the kappa value (0.25) of H. verticillata.
Although GAMs do not provide superior predictive performance compared with other models such as multivariate adaptive regression splines and boosted regression trees (Austin, 2007), they are flexible enough to model relationships between occurrences of submerged macrophytes and environmental factors (Murase et al., 2009). Habitats for submerged macrophytes are characterized by a complex set of physical, chemical, and biological parameters. In this study, the modeling of potential habitats for submerged macrophytes was in good agreement, despite only considering water chemicals, water depth, and water velocity. However, a reasonable possibility of prediction errors is the reason why the realized niches of submerged macrophytes were not completely explained by the variables that we selected and added to GAMs. We measured water depth and water velocity once in normal conditions, and we used water chemical data that were averaged over four years. Even though a lack of long-term monitoring data for water depth and velocity at the study sites is a source of uncertainty, we were able to overcome uncertainties by surveying diverse environmental conditions, varying from tributaries to rivers.
Buchan and Padilla (2000) found that the most important factors affecting the presence–absence of submerged macrophytes were associated with water quality rather than with dispersal potential. Nonetheless, to improve the predictive accuracy, it is also necessary to consider physical factors, such as flow regime, channel connectivity, channel slope, channel bed, shoreline conditions, and land cover type in the basin (Buchan and Padilla, 2000; Patrick et al., 2014). Biological factors, including competition, herbivory, and disease, are also important habitat determinants (Lacoul and Freedman, 2006). No habitat suitability model is a complete representation of reality and these models should be validated for applications using real-world data by predictive performance evaluations focusing on the reduction of omission errors (Liu et al., 2009; Gastón and García-Viñas, 2013). The distribution of M. spicatum and H. verticillata with high invasiveness could not be compared before and after water regulation in South Korea; however, we are able to predict the distribution using water quality data and adapt the models throughout the world.
4.3 Perspectives
We inferred the habitat characteristics of M. spicatum and H. verticillata using GAMs based on field survey data at the catchment scale. We observed that water chemicals, e.g., chlorophyll a, suspended solids, and nitrate nitrogen, water temperature, and electrical conductivity are important factors determining the occurrences of submerged macrophytes. Moreover, we validated the developed models by applying independent field data. Our research has practical implications for the prevention or delay of the aggressive spread of M. spicatum and H. verticillata by providing a basis for river management strategies, such as information about water chemicals to improve water quality in priority areas (Barko et al., 1986). These results are also helpful to sustain aquatic ecosystem functions and biodiversity in regulated hydrological conditions by identifying priority areas for monitoring and management.
Supplementary Material
Supplementary Tables. Access here
References
- Ahmadi-Nedushan B, St-Hilaire A, Bérubé M, Robichaud É, Thiémonge N, Bobée B. 2006. A review of statistical methods for the evaluation of aquatic habitat suitability for instream flow assessment. River Res Appl 22: 503–523. [CrossRef] [Google Scholar]
- Ali MM, Soltan MA. 2006. Expansion of Myriophyllum spicatum (Eurasian water milfoil) into Lake Nasser, Egypt: invasive capacity and habitat stability. Aquat Bot 84: 239–244. [CrossRef] [Google Scholar]
- Allouche O, Tsoar A, Kadmon R. 2006. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43: 1223–1232. [CrossRef] [Google Scholar]
- Angradi TR, Pearson MS, Bolgrien DW, Bellinger BJ, Starry MA, Reschke C. 2013. Predicting submerged aquatic vegetation cover and occurrence in a Lake Superior estuary. J Great Lakes Res 39: 536–546. [CrossRef] [Google Scholar]
- Araújo MB, Luoto M. 2007. The importance of biotic interactions for modelling species distributions under climate change. Glob Ecol Biogeogr 16: 743–753. [CrossRef] [Google Scholar]
- Austin MP. 2002. Spatial prediction of species distribution: an interface between ecological theory and statistical modelling. Ecol Model 157: 101–118. [CrossRef] [Google Scholar]
- Austin MP. 2007. Species distribution models and ecological theory: a critical assessment and some possible new approaches. Ecol Model 200: 1–19. [CrossRef] [Google Scholar]
- Austin MP, Meyers JA. 1996. Current approaches to modelling the environmental niche of eucalypts: implication for management of forest biodiversity. For Ecol Manage 85: 95–106. [CrossRef] [Google Scholar]
- Barko J, Adams M, Clesceri N. 1986. Environmental factors and their consideration in the management of submersed aquatic vegetation: a review. J Aquat Plant Manage 24: 1–10. [Google Scholar]
- Beck KG, Zimmerman K, Schardt JD, et al. 2008. Invasive species defined in a policy context: recommendations from the Federal Invasive Species Advisory Committee. Invasive Plant Sci Manage 1: 414–421. [CrossRef] [Google Scholar]
- Benda L, Andras K, Miller D, Bigelow P. 2004. Confluence effects in rivers: interactions of basin scale, network geometry, and disturbance regimes. Water Resour Res 40: W05402. [CrossRef] [Google Scholar]
- Borchers DL, Buckland ST, Priede IG, Ahmadi S. 1997. Improving the precision of the daily egg production method using generalized additive models. Can J Fish Aquat Sci 54: 2727–2742. [CrossRef] [Google Scholar]
- Bornette G, Puijalon S. 2011. Response of aquatic plants to abiotic factors: a review. Aquat Sci 73: 1–14. [CrossRef] [Google Scholar]
- Bučas M, Bergström U, Downie A-L, et al. 2013. Empirical modelling of benthic species distribution, abundance, and diversity in the Baltic Sea: evaluating the scope for predictive mapping using different modelling approaches. ICES J Mar Sci 70: 1233–1243. [CrossRef] [Google Scholar]
- Buchan LAJ, Padilla DK. 2000. Predicting the likelihood of Eurasian watermilfoil presence in lakes, a macrophyte monitoring tool. Ecol Appl 10: 1442–1455. [CrossRef] [Google Scholar]
- Buisson L, Blanc L, Grenouillet G 2008. Modelling stream fish species distribution in a river network: the relative effects of temperature versus physical factors. Ecol Freshwater Fish 17: 244–257. [CrossRef] [Google Scholar]
- Bunn SE, Arthington AH. 2002. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ Manage 30: 492–507. [Google Scholar]
- Burnham K, Anderson D. 1998. Model selection and inference: a practical information-theoretic approach. New York: Springer. [Google Scholar]
- Camporeale C, Ridolfi L. 2006. Riparian vegetation distribution induced by river flow variability: a stochastic approach. Water Resour Res 42: W10415. [CrossRef] [Google Scholar]
- Canfield D, Langeland K, Linda S, Haller W. 1985. Relations between water transparency and maximum depth of macrophyte colonization in lakes. J Aquat Plant Manage 23: 25–28. [Google Scholar]
- Chambers PA, DeWreede RE, Irlandi EA, Vandermeulen H. 1999. Management issues in aquatic macrophyte ecology: a Canadian perspective. Can J Bot 77: 471–487. [Google Scholar]
- Chappuis E, Gacia E, Ballesteros E. 2014. Environmental factors explaining the distribution and diversity of vascular aquatic macrophytes in a highly heterogeneous Mediterranean region. Aquat Bot 113: 72–82. [CrossRef] [Google Scholar]
- Cheng YW, Gallinat MP. 2004. Statistical analysis of the relationship among environmental variables, inter-annual variability and smolt trap efficiency of salmonids in the Tucannon River. Fish Res 70: 229–238. [CrossRef] [Google Scholar]
- Clayton J, Edwards T. 2006. Aquatic plants as environmental indicators of ecological condition in New Zealand lakes. Hydrobiologia 570: 147–151. [CrossRef] [Google Scholar]
- Cook CDK, Lüönd R. 1982. A revision of the genus Hydrilla (Hydrocharitaceae). Aquat Bot 13: 485–504. [CrossRef] [Google Scholar]
- Dar NA, Pandit AK, Ganai BA. 2014. Factors affecting the distribution patterns of aquatic macrophytes. Limnol Rev 14: 75–81. [Google Scholar]
- Dawson FH, Raven PJ, Gravelle MJ. 1999. Distribution of the morphological groups of aquatic plants for rivers in the U.K. In Caffrey J, Barrett PRF, Ferreira MT, Moreira IS, Murphy KJ, Wade PM, eds. Biology, Ecology and Management of Aquatic Plants: Proceedings of the 10th International Symposium on Aquatic Weeds, European Weed Research Society. Dordrecht: Springer Netherlands, pp. 123–130. [CrossRef] [Google Scholar]
- Dennison WC, Orth RJ, Moore KA, et al. 1993. Assessing water quality with submersed aquatic vegetation. Bioscience 43: 86–94. [CrossRef] [Google Scholar]
- Dodkins IAN, Rippey B, Hale P. 2005. An application of canonical correspondence analysis for developing ecological quality assessment metrics for river macrophytes. Freshw Biol 50: 891–904. [CrossRef] [Google Scholar]
- Downie AL, von Numers M, Boström C. 2013. Influence of model selection on the predicted distribution of the seagrass Zostera marina. Estuar Coast Shelf Sci 121: 8–19. [CrossRef] [Google Scholar]
- Drexler M, Ainsworth CH. 2013. Generalized additive models used to predict species abundance in the Gulf of Mexico: an ecosystem modeling tool. PLoS ONE 8: e 64458. [CrossRef] [PubMed] [Google Scholar]
- Dynesius M, Nilsson C. 1994. Fragmentation and flow regulation of river systems in the Northern Third of the World. Science 266: 753–762. [CrossRef] [PubMed] [Google Scholar]
- Eiswerth ME, Donaldson SG, Johnson WS. 2000. Potential environmental impacts and economic damages of Eurasian watermilfoil (Myriophyllum spicatum) in Western Nevada and Northeastern California. Weed Technol 14: 511–518. [CrossRef] [EDP Sciences] [Google Scholar]
- Elith J, Graham CH, Anderson RP, et al. 2006. Novel methods improve prediction of species' distributions from occurrence data. Ecography 29: 129–151. [CrossRef] [EDP Sciences] [Google Scholar]
- Fielding AH, Bell JF. 1997. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv 24: 38–49. [CrossRef] [Google Scholar]
- Franklin P, Dunbar M, Whitehead P. 2008. Flow controls on lowland river macrophytes: a review. Sci Total Environ 400: 369–378. [CrossRef] [PubMed] [Google Scholar]
- Freeman EA, Moisen GG. 2008. A comparison of the performance of threshold criteria for binary classification in terms of predicted prevalence and kappa. Ecol Model 217: 48–58. [CrossRef] [Google Scholar]
- Gallego I, Pérez-Martínez C, Sánchez-Castillo PM, Fuentes-Rodríguez F, Juan M, Casas JJ. 2015. Physical, chemical, and management-related drivers of submerged macrophyte occurrence in Mediterranean farm ponds. Hydrobiologia 762: 209–222. [CrossRef] [Google Scholar]
- Gassmann A, Cock MJW, Shaw R, Evans HC. 2006. The potential for biological control of invasive alien aquatic weeds in Europe: a review. Hydrobiologia 570: 217–222. [CrossRef] [Google Scholar]
- Gastón A, García-Viñas JI. 2013. Evaluating the predictive performance of stacked species distribution models applied to plant species selection in ecological restoration. Ecol Model 263: 103–108. [CrossRef] [Google Scholar]
- Guisan A, Edwards Jr TC, Hastie T. 2002. Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol Model 157: 89–100. [CrossRef] [Google Scholar]
- Heegaard E, Birks HH, Gibson CE, Smith SJ, Wolfe-Murphy S. 2001. Species-environmental relationships of aquatic macrophytes in Northern Ireland. Aquat Bot 70: 175–223. [CrossRef] [Google Scholar]
- Järvelä J. 2005. Effect of submerged flexible vegetation on flow structure and resistance. J Hydrol 307: 233–241. [CrossRef] [Google Scholar]
- Jiménez-Valverde A, Lobo JM. 2007. Threshold criteria for conversion of probability of species presence to either-or presence-absence. Acta Oecol 31: 361–369. [CrossRef] [Google Scholar]
- Jones RC, Walti K, Adams MS. 1983. Phytoplankton as a factor in the decline of the submersed macrophyte Myriophyllum spicatum L. in Lake Wingra, Wisconsin, USA. Hydrobiologia 107: 213–219. [CrossRef] [Google Scholar]
- Jun KS, Kim JS. 2011. The four major rivers restoration project: impacts on river flows. KSCE J Civ Eng 15: 217–224. [CrossRef] [EDP Sciences] [Google Scholar]
- Kennedy TL, Horth LA, Carr DE. 2009. The effects of nitrate loading on the invasive macrophyte Hydrilla verticillata and two common, native macrophytes in Florida. Aquat Bot 91: 253–256. [CrossRef] [Google Scholar]
- Kenneth AL. 1996. Hydrilla verticillata (L.F.) Royle (Hydrocharitaceae), “The Perfect Aquatic Weed”. Castanea 61: 293–304. [Google Scholar]
- Kiffney PM, Greene CM, Hall J, Davies J. 2006. Tributary streams create spatial discontinuities in habitat, biological productivity, and diversity in mainstem rivers. Can J Fish Aquat Sci 63: 2518–2530. [CrossRef] [Google Scholar]
- Klippel S, Amaral S, Vinhas L. 2016. Development and evaluation of species distribution models for five endangered elasmobranchs in southwestern Atlantic. Hydrobiologia 779: 11–33. [CrossRef] [Google Scholar]
- Kuhn M, Johnson K. 2013. Applied predictive modeling. New York: Springer. [Google Scholar]
- Lacoul P, Freedman B. 2006. Environmental influences on aquatic plants in freshwater ecosystems. Environ Rev 14: 89–136. [CrossRef] [Google Scholar]
- Lah T, Park Y, Cho YJ. 2015. The four major rivers restoration project of South Korea: an assessment of its process, program, and political dimensions. J Environ Dev 24: 375–394. [CrossRef] [Google Scholar]
- Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33 159–174. [CrossRef] [MathSciNet] [PubMed] [Google Scholar]
- Lathrop RG, Styles RM, Seitzinger SP, Bognar JA. 2001. Use of GIS mapping and modeling approaches to examine the spatial distribution of seagrasses in Barnegat Bay, New Jersey. Estuaries 24: 904–916. [CrossRef] [Google Scholar]
- Lauridsen TL, Jeppesen E, Declerck SAJ, et al. 2015. The importance of environmental variables for submerged macrophyte community assemblage and coverage in shallow lakes: differences between northern and southern Europe. Hydrobiologia 744: 49–61. [CrossRef] [Google Scholar]
- Lee JW, Bae SI, Lee DR, Seo DI. 2014. Transportation modeling of conservative pollutant in a river with weirs − the Nakdong River case. J Korean Soc Environ Eng 36: 821–827. [CrossRef] [Google Scholar]
- Lehmann A. 1998. GIS modeling of submerged macrophyte distribution using Generalized Additive Models. Plant Ecol 139: 113–124. [CrossRef] [Google Scholar]
- Li X, Wang Y. 2013. Applying various algorithms for species distribution modelling. Integr Zool 8: 124–135. [CrossRef] [PubMed] [Google Scholar]
- Liu C, White M, Newell G. 2009. Measuring the accuracy of species distribution models: a review. In: Proceedings 18th World IMACs/MODSIM Congress , Cairns, Australia, pp. 4241–4247. [Google Scholar]
- Murase H, Nagashima H, Yonezaki S, Matsukura R, Kitakado T. 2009. Application of a generalized additive model (GAM) to reveal relationships between environmental factors and distributions of pelagic fish and krill: a case study in Sendai Bay, Japan. ICES J Mar Sci 66: 1417–1424. [CrossRef] [Google Scholar]
- Narumalani S, Jensen JR, Burkhalter S, Althausen JD, Mackey Jr HE. 1997. Aquatic macrophyte modeling using GIS and logistic multiple regression. Photogramm Eng Remote Sens 63: 41–49. [Google Scholar]
- Nepf H, Ghisalberti M, White B, Murphy E. 2007. Retention time and dispersion associated with submerged aquatic canopies. Water Resour Res 43: W04422. [CrossRef] [Google Scholar]
- Netherland MD. 1997. Turion ecology of hydrilla. J Aquat Plant Manage 35: 1–10. [Google Scholar]
- Nichols SA, Shaw BH. 1986. Ecological life histories of the three aquatic nuisance plants, Myriophyllum spicatum, Potamogeton crispus and Elodea canadensis. Hydrobiologia 131: 3–21. [CrossRef] [Google Scholar]
- Nieder WC, Barnaba E, Findlay SEG, Hoskins S, Holochuck N, Blair EA. 2004. Distribution and abundance of submerged aquatic vegetation and Trapa natans in the Hudson River Estuary. J Coast Res 150–161. [CrossRef] [Google Scholar]
- Normile D. 2010. Restoration or devastation? Science 327: 1568–1570. [CrossRef] [PubMed] [Google Scholar]
- O'Hare JM, O'Hare MT, Gurnell AM, Dunbar MJ, Scarlett PM, Laizé C. 2011. Physical constraints on the distribution of macrophytes linked with flow and sediment dynamics in British rivers. River Res Appl 27: 671–683. [CrossRef] [EDP Sciences] [Google Scholar]
- Park HK, Cho KH, Won DH, Lee J, Kong DS, Jung DI. 2013. Ecosystem responses to climate change in a large on-river reservoir, Lake Paldang, Korea. Clim Change 120: 477–489. [CrossRef] [Google Scholar]
- Patrick CJ, Weller DE, Li X, Ryder M. 2014. Effects of shoreline alteration and other stressors on submerged aquatic vegetation in subestuaries of Chesapeake Bay and the Mid-Atlantic Coastal Bays. Estuar Coast 37: 1516–1531. [CrossRef] [Google Scholar]
- Pearce J, Ferrier S. 2000. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol Model 133: 225–245. [CrossRef] [Google Scholar]
- Peters J, Baets BD, Verhoest NEC, Samson R, Degroeve S, Becker PD, Huybrechts W. 2007. Random forests as a tool for ecohydrological distribution modelling. Ecol Model 207: 304–318. [CrossRef] [Google Scholar]
- QGIS Development Team. 2016. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://www.qgis.org/. [Google Scholar]
- R Development Core Team . 2016. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. http://www.r-project.org/. [Google Scholar]
- Rice SP, Ferguson RI, Hoey TB. 2006. Tributary control of physical heterogeneity and biological diversity at river confluences. Can J Fish Aquat Sci 63: 2553–2566. [CrossRef] [Google Scholar]
- Riis T, Biggs BJF. 2003. Hydrologic and hydraulic control of macrophyte establishment and performance in streams. Limnol Oceanogr 48: 1488–1497. [CrossRef] [Google Scholar]
- Riis T, Sand-Jensen K, Larsen SE. 2001. Plant distribution and abundance in relation to physical conditions and location within Danish stream systems. Hydrobiologia 448: 217–228. [CrossRef] [Google Scholar]
- Rybicki NB, Landwehr JM. 2007. Long-term changes in abundance and diversity of macrophyte and waterfowl populations in an estuary with exotic macrophytes and improving water quality. Limnol Oceanogr 52: 1195–1207. [CrossRef] [Google Scholar]
- Sanchez P, Demestre M, Recasens L, Maynou F, Martin P. 2008. Combining GIS and GAMs to identify potential habitats of squid Loligo vulgaris in the Northwestern Mediterranean. Hydrobiologia 612: 91–98. [CrossRef] [Google Scholar]
- Segurado P, Araujo MB. 2004. An evaluation of methods for modelling species distributions. J Biogeogr 31: 1555–1568. [CrossRef] [Google Scholar]
- Shin JH, Chung JY. 2011. The four major rivers restoration project in South Korea. Proc Inst Civ Eng Civ Eng 164: 19–26. [Google Scholar]
- Shmueli G. 2010. To explain or to predict? Stat Sci 289–310. [CrossRef] [Google Scholar]
- Sing T, Sander O, Beerenwinkel N, Lengauer T. 2005. ROCR: visualizing classifier performance in R. Bioinformatics 21: 3940–3941. [CrossRef] [PubMed] [Google Scholar]
- Solanki HU, Bhatpuria D, Chauhan P. 2016. Applications of generalized additive model (GAM) to satellite-derived variables and fishery data for prediction of fishery resources distributions in the Arabian Sea. Geocarto Int 32: 30–43. [CrossRef] [Google Scholar]
- Sousa W. 2011. Hydrilla verticillata (Hydrocharitaceae), a recent invader threatening Brazil's freshwater environments: a review of the extent of the problem. Hydrobiologia 669: 1–20. [CrossRef] [Google Scholar]
- Sousa WTZ, Thomaz SM, Murphy KJ, Silveira MJ, Mormul RP. 2009. Environmental predictors of the occurrence of exotic Hydrilla verticillata (L.f.) Royle and native Egeria najas Planch. in a sub-tropical river floodplain: the Upper River Paraná, Brazil. Hydrobiologia 632: 65–78. [CrossRef] [Google Scholar]
- Strand JA, Weisner SEB. 1996. Wave exposure related growth of epiphyton: implications for the distribution of submerged macrophytes in eutrophic lakes. Hydrobiologia 325: 113–119. [CrossRef] [Google Scholar]
- Swets J. 1988. Measuring the accuracy of diagnostic systems. Science 240: 1285–1293. [CrossRef] [PubMed] [Google Scholar]
- Thomaz SM, Souza DC, Bini LM. 2003. Species richness and beta diversity of aquatic macrophytes in a large subtropical reservoir (Itaipu Reservoir, Brazil): the influence of limnology and morphometry. Hydrobiologia 505: 119–128. [CrossRef] [Google Scholar]
- Thuiller W, Richardson DM, PyŠEk P, Midgley GF, Hughes GO, Rouget M. 2005. Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Glob Change Biol 11: 2234–2250. [CrossRef] [Google Scholar]
- Tian K, Liu G, Xiao D, Sun J, Lu M, Huang Y, Lin P. 2015. Ecological effects of dam impoundment on closed and half-closed wetlands in China. Wetlands 35: 889–898. [CrossRef] [Google Scholar]
- Van TK, Wheeler GS, Center TD. 1999. Competition between Hydrilla verticillata and Vallisneria americana as influenced by soil fertility. Aquat Bot 62: 225–233. [CrossRef] [Google Scholar]
- VanDerWal J, Falconi L, Januchowski S, Shoo L, Storlie C. 2014. SDMTools: Species Distribution Modelling tools: tools for processing data associated with species distribution modelling exercises. R package version, 1.1-221. [Google Scholar]
- Wedding L, Yoklavich MM. 2015. Habitat-based predictive mapping of rockfish density and biomass off the central California coast. Mar Ecol Prog Ser 540: 235–250. [CrossRef] [Google Scholar]
- Woo H. 2010. Trends in ecological river engineering in Korea. J Hydroenviron Res 4: 269–278. [Google Scholar]
- Wood SN. 2000. Modelling and smoothing parameter estimation with multiple quadratic penalties. J R Stat Soc Ser B (Stat Method) 62: 413–428. [CrossRef] [Google Scholar]
- Wood SN. 2006. Generalized additive models: an introduction with R. Boca Raton: CRC Press. [Google Scholar]
- Xie D, Yu D, You WH, Wang LG. 2013. Morphological and physiological responses to sediment nutrients in the submerged macrophyte Myriophyllum spicatum. Wetlands 33: 1095–1102. [CrossRef] [Google Scholar]
- Yee TW, Mitchell ND. 1991. Generalized additive models in plant ecology. J Veg Sci 2: 587–602. [CrossRef] [Google Scholar]
- Yu H, Ye C, Song X, Liu J. 2010. Comparative analysis of growth and physio-biochemical responses of Hydrilla verticillata to different sediments in freshwater microcosms. Ecol Eng 36: 1285–1289. [CrossRef] [Google Scholar]
- Zhao J, Cao J, Tian S, Chen Y, Zhang S, Wang Z, Zhou X. 2014. A comparison between two GAM models in quantifying relationships of environmental variables with fish richness and diversity indices. Aquat Ecol 48: 297–312. [CrossRef] [Google Scholar]
- Zhou N, Hu W, Deng J, Zhu J, Xu W, Liu X. 2016. The effects of water depth on the growth and reproduction of Potamogeton crispus in an in situ experiment. J Plant Ecol 10: 546–558. [Google Scholar]
- Zimmermann NE, Edwards TC, Moisen GG, Frescino TS, Blackard JA. 2007. Remote sensing-based predictors improve distribution models of rare, early successional and broadleaf tree species in Utah. J Appl Ecol 44: 1057–1067. [CrossRef] [PubMed] [Google Scholar]
- Zuur AF, Pierce GJ. 2004. Common trends in northeast Atlantic squid time series. J Sea Res 52: 57–72. [CrossRef] [Google Scholar]
Cite this article as: Son D, Cho K-H, Lee EJ. 2017. The potential habitats of two submerged macrophytes, Myriophyllum spicatum and Hydrilla verticillata in the river ecosystems, South Korea. Knowl. Manag. Aquat. Ecosyst., 418, 58.
All Tables
Mean, standard error (SE), minimum (Min), and maximum (Max) values for physical and chemical properties of water at 197 sites. Chlorophyll a, electrical conductivity, total dissolved phosphorus, nitrate nitrogen, and suspended solids are presented as mean values of monthly estimates from 2012 to 2015, water temperature is presented as the mean from May to October, and water depth and velocity data were obtained at the sampling date.
Selected environmental variables and deviance explained in GAMs for Myriophyllum spicatum and Hydrilla verticillata.
Comparison of predicted and observed distributions of Myriophyllum spicatum and Hydrilla verticillata during model building. Predicted values were obtained from the fitted probability of presence using the Youden index to apply a threshold probability: 0.536 for M. spicatum and 0.492 for H. verticillata.
Comparison of predicted and observed distributions of Myriophyllum spicatum and Hydrilla verticillata at the model confirmation stage. Predicted values were obtained from the fitted probability of presence using the Youden index to apply a threshold probability: 0.536 for M. spicatum and 0.492 for H. verticillata.
All Figures
 |
Fig. 1 Studied streams in South Korea. The streams were roughly classified into five rivers: Han River, Geum River, Nakdong River, Yeongsan River, and Seomjin River. |
| In the text | |
 |
Fig. 2 Response curves of (A) Myriophyllum spicatum and (B) Hydrilla verticillata for environmental gradients in GAMs. The vertical axes represent the probabilities of occurrence, and shaded bands show the 95% confidence interval. Rug plots on the x-axis show data points. Chla, chlorophyll a; EC, electrical conductivity; NO3N, nitrate nitrogen; SS, suspended solids; WT, water temperature; WD, water depth; WV, water velocity. |
| In the text | |
 |
Fig. 3 Predicted and observed habitat suitability of (A) Myriophyllum spicatum and (B) Hydrilla verticillata based on GAMs. Observed occurrence (Ob.) is overlaid with the predicted occurrence (Pr.). The outer circle indicates the observed distribution, and the inner circle represents the predicted distribution. Yellow indicates presence (1) and black indicates absence (0). The same color for the outer and inner circles shows that observations and predictions coincide. A black outer circle with a yellow inner circle indicates a false positive, and a yellow outer with a black inner circle indicates a false negative. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


