| Issue |
Knowl. Manag. Aquat. Ecosyst.
Number 420, 2019
|
|
|---|---|---|
| Article Number | 5 | |
| Number of page(s) | 14 | |
| DOI | https://doi.org/10.1051/kmae/2018044 | |
| Published online | 14 February 2019 | |
Research Paper
Phytoplankton functional group classifications as a tool for biomonitoring shallow lakes: a case study
Les groupes fonctionnels du phytoplancton en tant qu'outils de biosurveillance des lacs urbains : une étude de cas
1
Instituto del Conurbano, Universidad Nacional de General Sarmiento, Juan María Gutiérrez 1150, Los Polvorines CP B1613, Buenos Aires, Argentina
2
Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina
3
Instituto Multidisciplinario sobre Ecosistemas y Desarrollo Sustentable, Universidad Nacional del Centro de la Provincia de Buenos Aires, Buenos Aires, Argentina
4
Instituto de Ecología, Genética y Evolución de Buenos Aires, Universidad de Buenos Aires, Buenos Aires, Argentina
* Corresponding author: lallende@campus.ungs.edu.ar
We assessed changes in phytoplankton community structure in relation to environmental variables in an urban eutrophic shallow lake (Lake Lugano, Argentina) throughout one year following two classification systems according to the morpho-functional groups (MFG) approach and morphologically based functional groups (MBFG). We aimed to compare the different approaches and find a simple tool to biomonitor urban freshwaters regarding their phytoplankton structure. Values of transparency, nutrients and chlorophyll a concentrations confirmed the eutrophic/hypertrophic conditions of the lake. The potentially toxic Cyanobacteria Planktothrix agardhii representing MFG 5a and MBFG III was generally dominant and reached bloom densities (>62,000 ind ml−1). The multivariate analyses performed showed similar and overlapping results considering both approaches. Nutrients and transparency were the main environmental variables explaining the variance encountered. We conclude that MBFG classification was an adequate, easy-to-handle method for monitoring Lake Lugano. The functional approaches applied enabled the follow-up of potentially toxic Cyanobacteria in Lake Lugano. Further studies should include the estimation of cyanobacteria-derived toxin concentrations in water. We consider that the applicability of the MBFG approach deserves to be further explored as a promising tool for biomonitoring different types of urban water bodies.
Résumé
Nous avons évalué les changements dans la structure de la communauté phytoplanctonique par rapport aux variables environnementales dans un lac peu profond eutrophique urbain (lac Lugano, Argentine) tout au long d'une année selon deux systèmes de classification ; l'approche morpho-functional groups (MFG) et morphologically based functional groups (MBFG). Nous avons cherché à comparer les différentes approches et à trouver un outil simple pour surveiller les eaux douces urbaines en ce qui concerne leur structure phytoplanctonique. Les valeurs de transparence, de nutriments et de concentrations de chlorophylle a ont confirmé les conditions eutrophiques/hypertrophiques du lac. Les cyanobactéries Planktothrix agardhii potentiellement toxiques représentant MFG 5a et MBFG III étaient généralement dominantes et ont atteint des densités d'efflorescence (>62,000 ind ml−1). Les analyses multivariées effectuées ont donné des résultats similaires, se chevauchant pour les deux approches. Les nutriments et la transparence étaient les principales variables environnementales expliquant la variance rencontrée. Nous concluons que la classification MBFG était une méthode adéquate et facile à utiliser pour la surveillance du lac de Lugano. Les approches fonctionnelles appliquées ont permis le suivi des cyanobactéries potentiellement toxiques dans le lac de Lugano. D'autres études devraient inclure l'estimation des concentrations de toxines dérivées de cyanobactéries dans l'eau. Nous estimons que l'applicabilité de l'approche MBFG mérite d'être étudiée plus avant en tant qu'outil prometteur pour la biosurveillance de différents types de masses d'eau urbaines.
Key words: urbanization / eutrophication / Cyanobacteria / bloom / morpho-functional approach
Mots clés : urbanization / eutrophisation / Cyanobacteria / efflorescences algales / approximation morpho-fonctionnelle
© L. Allende et al., Published by EDP Sciences 2019
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (http://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (http://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
1 Introduction
In the near future more than 50% of the humans will concentrate in cities as a result of population growth and migration from rural areas (Shochat et al., 2006; WWAP, 2012). Urbanization is rapidly increasing and has become a matter of concern in conservation biology mainly due to the radical changes in habitat structure and the loss of endangered species by urban development. As deeply discussed by different authors, the term “urban” is difficult to define as it varies notably among countries. The definitions are based on human population density, legal municipal boundaries, degree of land use, and built areas (Pickett and Cadenasso, 2006; MacGregor-Fors, 2011; Francis and Chadwick, 2012). MacGregor-Fors (2011) recognizes the importance of homogenizing the term and re-defines the term “urban” considering that it refers to populated areas provided with basic services, where more than 1000 people/km2 live or work, and an important proportion of the land (>50%) in a “city-scale” is covered by impervious surfaces. Besides the current debate, there is no doubt that the urban habitat conforms a unique setting, in which fundamental biological trends and processes can be decoupled by antrophogenic activities.
Water bodies form part of the urban landscape and constitute an element of interaction with their inhabitants; they have different uses and their water quality comprises an important issue for the urban development. They include naturally formed lakes with surrounding development, artificial quarry lakes, and recreational park-ponds, which can be subjected to an increasing negative environmental impact, and their water quality is often under great pressure (Olding et al., 2000; Gledhill et al., 2008; Henny and Meutia, 2014). Freshwater ecosystems provide the society with numerous ecosystem services but it is now recognized that human alterations of freshwaters have decreased their value (Taranu et al., 2015). Cultural eutrophication of freshwater ecosystems is a problem worldwide, caused by increased nutrient loads resulting in water quality deterioration (Becklioglu et al., 2011; Araújo et al., 2016). In this sense, urban areas constitute important sources of chemicals for freshwater ecosystems (Carpenter et al., 2011). Eutrophication of urban ponds and shallow lakes is repeatedly reported in cities all around the world (Xiangcan, 2003; Fabre et al., 2010; Waajen et al., 2014; Yu et al., 2016; Rodríguez-Flores, 2017) and can be nowadays considered a mainstream in aquatic ecology. Even though the available information for them is relatively scarce if compared with that for pristine water bodies, different aspects of the biotic components of urban aquatic systems have lately achieved an increased interest from ecologists. In particular, phytoplankton-focused studies have flourished (e.g., Scasso et al., 2001; Xiangcan, 2003; Hirose et al., 2003; Meerhoff et al., 2003; Fazio and O'farrell, 2005; Lv et al., 2011; Segura et al., 2013; Avigliano et al., 2014; Waajen et al., 2014; Jovanović et al., 2017).
Pickett and Cadenasso (2006) stressed out that the flora and fauna of the metropolitan areas are mostly dominated by generalist species (e.g., early successional trees and herbs, and species that can tolerate disturbance). They also highlight that a still open research question lies in the functional role of species in the ecological processes of urban ecosystems. Understanding the mechanisms to predict changes in plant and animal communities is a key challenge in ecology (Blaum et al., 2011). In this sense, the need to transfer knowledge gained from single species to a more generalized approach has led to the development of categorization systems where species' similarities in life strategies and traits are classified into ecological groups (EGs) like functional groups/types or guilds. While approaches in plant ecology undergo a steady improvement and refinement of methodologies, and the progression in animal ecology is challenging (Blaum et al., 2011), there is a strong case for attempting to categorize the phytoplankton species on the basis of their functional roles in aquatic ecosystem (Reynolds, 2006; Salmaso et al., 2015). Phytoplankton is in general a highly diverse community and the occurrence of most species is hard to predict (Benincá et al., 2008; Kruk et al., 2011). Naselli-Flores and Barone (2011) suggested that a promising approach to solve this secret is the possibility to group phytoplankton species by means of their functional attributes. They also highlighted that trait-based classifications are increasingly used in phytoplankton ecology in order to explain and predict functional groups distributions along environmental gradients.
Recently, morphological and functional analyses of phytoplankton have become more and more popular. This fact mainly draws from the need to find easy-to-handle descriptors for management purposes (Naselli-Flores and Barone, 2011). When attempting to understand biodiversity response to environmental changes, the main advantage of applying functional groups classifications over single species approaches lies in the generalization of results (Blaum et al., 2011). Salmaso et al. (2015) examined nine modes of classifying phytoplankton nontaxonomically. The classification methods assessed were based on either functional, morphometric, and morphological criteria (Margalef, 1978; Platt and Denman, 1978; Reynolds, 1988a,b; Kamenir et al., 2004, 2006; Kruk et al., 2010; Stanca et al., 2013) or a combination of these with phenological, ecological, and/or taxonomic features when relevant (Reynolds, 1980; Reynolds et al., 2002; Salmaso and Padisak, 2007). From these combined classifications, a largely influential and accepted approach among phytoplankton ecologists is that of Reynoldś (Reynolds et al., 2002 updated by Padisák et al., 2009). This classification is based on the functional traits of phytoplankton species, the ranges of environmental conditions over which they are found, as well as their co-occurrence. The success of this classification probably derives from the simplification of long taxonomic lists into about three dozens of functional groups (FG) or coda, sharing similar ecological characteristics (Naselli-Flores and Barone, 2011). Nonetheless, it requires specialist judgment in order to recognize algae up to the species level and classify them into the different coda. A relatively simpler method is the morpho-functional group (MFG) classification proposed by Salmaso and Padisak (2007) and updated recently (Tolotti et al., 2012). This classification is based on morphological and structural features and includes taxonomy. It requires the ability to recognize the species from genus to order level and it is based on traits that strongly influence both ecological characteristics and functional processes. These authors based the MFGs on the criteria defined by Weithoff (2003) that are the presence of flagella, morphology (size, shape), ability to obtain alternative sources of nutrients and carbon, the presence of gelatinous envelopes, and taxonomy. As a result, phytoplankton species can be classified in 33 groups (after the subdivision proposed for diatoms by Tolotti et al., 2012). An alternative classification based purely on morphological descriptors was proposed by Kruk et al. (2010). These authors defined seven species groups based on cluster analyses; this morphologically based functional group classification (MBFG) relies on the fact that the selected observable morphological traits (volume, maximum linear dimension, surface area, among others) are tightly linked to the autoecology of planktonic algae. In this case, the classification results in much greater simplification, leading to seven different groups.
With this paper, we attempt to monitor the changes in the phytoplankton community structure in an urban shallow lake throughout one year following two classification systems: morpho-functional groups (Salmaso and Padisak, 2007, updated by Tolotti et al., 2012) and morphologically based functional groups (Kruk et al., 2010). The aim is to compare the different approaches in order to find a relatively simple tool to biomonitor lentic aquatic ecosystems located in urban areas on the basis of their phytoplankton structure. As well, we aim to identify the dominant phytoplankton species and also detect the presence of potentially toxic and /or bloom-forming algae, as they represent a relevant aspect to determine the water quality of systems in close relation to the humans. Our study supports the application of the ecological group approach in the assessment of the phytoplankton response to environmental changes. It reinforces the idea of the use of a simple and sensitive enough method to monitor urban aquatic systems.
2 Materials and methods
2.1 Study site
Parque Roca constitutes the largest recreational park within the city of Buenos Aires (Argentina) with an area of 160 hectares (Sinistro et al., 2004) (Fig. 1). It constitutes a green area surrounded by neighborhoods showing population densities greater than 10,371 inhabitants per km2 and even surpassing 59,173 inhabitants per km2, according to a recent population census (DGEC, 2016). Multiple activities (cultural, sportive, and social) take place in this park. It is an area with a high recreational value for the community. Lake Lugano is a man-made shallow water body (mean depth 1.5 m, maximum depth 3.0 m) located in this park. Its surface area is approximately 20 hectares, with a volume of 249.973 m3. This water body was constructed in the 1950s. The water supply mainly consists on that derived from rains and superficial runoff waters. However, it has received untreated wastewater in an illegal manner during a long period (Rodríguez et al., 2003), leading to the deterioration of its water quality. At the moment, the use of this water body for aquatic recreational activities is forbidden.
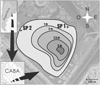 |
Fig. 1 Location of Lake Lugano in Parque Roca (Buenos Aires, Argentina), and the respective sampling points (SP1 and SP2). |
2.1.1 Environmental variables
Lake Lugano was monitored monthly during a 1-year period (28/5/2015 to 9/6/2016). Two sampling points (SP1 and SP2) were established in the littoral area based on previous monitoring studies held on this water body (Rodríguez et al., 2003; Sinistro et al., 2004). These sites were selected as they are highly accessible to the visitors and thus, represent areas of increased chances of contact between the public and the waters of the shallow lake. Environmental variables were measured in situ with portable instruments: pH, water temperature and conductivity (HANNA HI9811-5), dissolved oxygen (YSI ProODO), and turbidity (HACH 2100P). Transparency was estimated with a Secchi disc. Water samples were collected in acid-washed PVC bottles for the determination of the concentration of nutrients: ammonia (NH4), nitrates (N-NO3), dissolved reactive phosphorus (DRP), total nitrogen (TN), and total phosphorus (TP). Dissolved nutrients were analyzed after sample filtration through GF/F Whatman™ glass-fiber filters as follows: NH4 with the phenate method, N-NO3 with the cadmium reduction method, and DRP with the molybdovanadate method (APHA, 2005) using Hach® reagents. Total phosphorus (TP) and nitrogen (TN) were determined from unfiltered samples and posterior oxidation following Valderrama (1981). Daily air temperature and rainfall were provided by the Servicio Meteorológico Nacional (Argentina).
The phytoplanktonic chlorophyll a concentration was estimated following Marker et al. (1980). Water samples were obtained subsuperficially and filtered through GF/F Whatman™ glass-fiber filters. Samples for the study of the phytoplankton structure and dynamics were collected as follows: for the qualitative analysis, water samples were obtained using a 15 μm phytoplankton net, fixed with 5% formalin and observed with a Zeiss™ optical microscope at ×1000 magnification. Quantitative samples were obtained subsuperficially and fixed with acetic Lugol's solution. Phytoplankton counts were performed using a Zeiss™ inverted microscope (Ütermöhl, 1958) and sedimentation chambers (2–5 ml sample). The counting error was estimated according to Venrick (1978). Individual algae (independently if it presented a colonial, a filamentous, cenobial, or unicellular habit) were considered as the unit. The phytoplankton organisms encountered throughout the study period were assigned to different ecological groups. We considered the MFG classification by Salmaso and Padisak (2007), considering potential mixotrophy, size (small <30–40 μm/large >30–40 μm), shape, presence of gelatinous envelopes, specific nutrient requirements and motility, and taxonomy when ecologically relevant. As for the MBFG, the classification was performed considering the presence/absence of flagella, siliceous structures, aerotopes, and/or mucilaginous envelopes, as well as morphometrical parameters (Kruk et al., 2010).
2.1.2 Data analysis
Spearman's rank nonparametric correlations were performed between pairs of environmental variables. To characterize the structure of phytoplankton assemblages, we estimated the Shannon Diversity Index (H) and evenness (J) (based on Shannon index) for each sampling site and date, and functional group classifications. Moreover, we estimated the Bray–Curtis Similarity Index to evaluate the similitude between sites on each sampling date and both classification methods (Zar, 1999). The analyses were performed with Past 3.14 (Hammer et al., 2001).
To assess for significant relationships between biological and environmental data, direct ordination analyses were used. First, a detrended correspondence analysis (DCA) was performed and, as data showed a linear response, a redundancy analysis (RDA) was applied. Environmental parameters that were highly correlated (r > 0.7) and with an inflation factor >10 were excluded from the analysis. MFG and MBFG were considered as the response variables and evaluated separately. Forward analysis of variables was used in all cases, considering the explained percentage of the variance and its significance. The Monte Carlo test was used for the statistical validation of the association between the ordination values of species, samples, and environmental variables. A total of 499 iterations were done for this test and considered to be significant at p < 0.05 level (ter Braak and Verdonschot, 1995). Multivariate analyses were performed using the software CANOCO 4.5.
3 Results
3.1 Environmental variables
Table 1 summarizes the ranges encountered for different physical and chemical variables throughout an annual cycle (late-autumn 2015/late-autumn 2016) for the studied urban water body. Lake Lugano presented a strong seasonal variability in water temperature ranging from 10.20 to 28.30 °C. The lowest temperatures were observed during late-autumns (2015 and 2016) and the highest during the summer, showing a clear seasonal pattern (Fig. 2a) and a high correlation with daily mean air temperature (r = 0.910, p < 0.0001). Conductivity values were relatively high and presented minimal differences between sampling sites, with the exception of the peak registered for SP2 (Fig. 2b) during April 2016. It was inversely correlated (r = −0.525, p = 0.008) with pluvial precipitation accumulated in the previous week (PP PW) of sampling date. As for pH, values remained alkaline during the whole studied period with a minimum value of pH = 8. The highest value registered (pH = 9.5) coincided with the warmer temperatures. SP1 presented higher values than SP2 during almost the whole studied period (Fig. 2c). Dissolved oxygen (as well as oxygen percentage saturation) strongly fluctuated throughout the annual cycle, showing concentrations as low as 0.21 mg L−1 (representing 2.6% saturation) up to supersaturation values 20.65 mg L−1 (representing 265% saturation), SP1 generally presenting higher concentrations as compared with SP2 (Fig. 2d). Secchi depth was generally low (<40 cm), only exceeding that value during the cold months (Fig. 2e). This variable and conductivity were inversely correlated (r = −0.629, p = 0.001).
Nutrient concentrations presented marked variations (Fig. 3), mainly in the nitrogenous forms. As for the reduced nitrogen form (NH4), figures were as dissimilar as two orders of magnitude (Fig. 3a). In general terms, the highest concentrations for this nutrient were estimated at the end of the studied period in the cold season (Austral autumn, March–June 2016). The oxidized nitrogen form (N-NO3) followed a similar trend (Fig. 3b) with lowest values during the warmer season. NH4 and N-NO3 concentrations were positively correlated (r = 0.832, p < 0.0001). DRP presented a rather similar variation at both sampling points (Fig. 3c), and a positive correlation with turbidity (r = 0.622, p = 0.003). TP and TN concentrations oscillated between 1.80–6.48 mg L−1 and 1.32–3.84 mg L−1, showing sharp fluctuations throughout the studied annual cycle (Fig. 3d and 3e). TP was positively correlated with water temperatures (r = 0.592, p = 0.002).
Ranges encountered for physical and chemical parameters in Lake Lugano throughout the studied period 2015–2016 (SD: standard deviation, min: minimum, max: maximum). Turbid: turbidity, NH4: ammonia, N-NO3: nitrates, TN: total nitrogen, DRP: dissolved reactive phosphorus, TP: total phosphorus, Cond.: conductivity, Water Temp.: water temperature, DO: dissolved oxygen, Secchi: Secchi transparency, Chlor a: phytoplanctonic chlorophyll a concentration, SP: sampling point.
 |
Fig. 2 Environmental variables measured in Lake Lugano during the annual cycle 2015–2016. (a) Water temperature. (b) Conductivity. (c) pH. (d) Dissolved oxygen concentration. (e) Transparency. SP: sampling point. |
 |
Fig. 3 Fluctuations in nutrient concentrations throughout the studied period in Lake Lugano. (a) NH4: ammonia, (b) N-NO3: nitrates, (c) DRP: dissolved reactive phosphorus, (d) TP: total phosphorus, (e) TN: total nitrogen. SP: sampling point. |
3.2 Phytoplanktonic community
Phytoplanktonic chlorophyll a concentrations differed strongly throughout the study, evidencing a variation of three orders of magnitude considering the maximum value 235.30 μg L−1–(July 2015) and its minimum 0.51 μg L−1 (June 2016). The pattern of fluctuation was similar at SP1 and SP2, showing four and three clearly defined peaks (Fig. 4). Both, DRP concentration and turbidity were positively correlated with the chlorophyll a concentration (r = 0.768, p < 0.0001; r = 0.768, p < 0.0001, respectively).
Independent of the phytoplankton classification adopted (MFG or MBFG), the patterns of fluctuation of the community parameters, such as biodiversity or evenness, were very similar almost all throughout the study period and for both SP1 and SP2 (Fig. 5a and 5b). Moreover, Bray–Curtis similarity index revealed a very strong similitude assemblage (>0.8) composition between both sites almost at all sampling dates (Fig. 5c) whatever the classification approach was chosen. An exception was detected on spring 2015 and late-summer 2016 when this index was less than 50% (0.4). The Shannon Index never exceeded 1.6 and reached very low values (<0.2) during late-winter and spring 2015, mostly at SP1. The evenness associated to this index only presented high values (>0.8) occasionally and three clearly distinguishable peaks at autumn 2015 (May), summer (February 2016), and autumn again (June 2016). The autumn peaks coincided with moments of highest and minimum phytoplankton densities.
Phytoplankton density showed strong fluctuations at different moments throughout the study period oscillating between 92,639 and 707 ind ml−1 (Fig. 6a–6d), showing maximum and minimum values during the autumn seasons 2015 and 2016, respectively. After classifying the phytoplankton following the different approaches, it was observed that considering the MFG classification, the phytoplankton was generally dominated by MFG 5a (characterized by Cyanobacteria − filamentous Oscillatoriales) all throughout the annual cycle with the exception of late-autumn 2015, February 2016, and late autumn 2016 (Fig. 6a and 6b). However, it is worth to mention that even though this group was not dominant during autumn 2015, its densities were very high, reaching 26,660 ind ml−1 and 29,880 ind ml−1 at SP1 and SP2, respectively. At that moment, the MFGs co-dominating Lake Lugano's phytoplankton were MFG 5a and MFG 3a (characterized by autotrophic unicellular flagellates Phytomonadina), followed by MFG 7a (small centric diatoms). On the contrary, and for the same season in 2016, MFG 5a densities strongly dropped achieving minimum values at SP2 (22 ind ml−1) and not being registered at SP1. February 2016 presented a phytoplankton community mainly represented by four MFG with very similar and relatively low densities each with an even structure (SP1 J = 0.78; SP2 J = 0.71), namely, MFG 5a, MFG 6a1 (characterized by large >30 μm − unicellular centric diatoms), MFG 11a (nonfilamentous chlorococcalean naked colonies), and MFG 11b (nonfilamentous chlorococcalean gelatinous colonies). The MFG dynamics throughout the studied period was very similar for both SP1 and SP2. Regarding the MBFG classification, a very similar pattern in group fluctuation was registered throughout the annual cycle, as compared with MFG (Fig. 6c and 6d). MBFG III (characterized by large filaments of Nostocales and Oscillatoriales with aerotopes) and MBFG V (represented by unicellular flagellates of medium to large size) were co-dominant during late-autumn 2015. After this date, a single group dominated the phytoplankton community at both study sites with the exception of February 2016 and autumn 2016. In this respect, MFBG III represented more than 80% of the total density during winter, spring 2015, and almost all throughout the summer. Again, for February 2016, an even phytoplankton structure was encountered (SP1 J = 1.0; SP2 J = 0.87). During this sampling date, MBFG III, MBFG IV (organisms of medium size lacking specialized structures), MBFG VI (nonflagellated organisms with siliceous exoskeletons), and MBFG VII (large mucilaginous colonies) represented the phytoplankton community at both sampling sites.
It is worth mentioning that the bloom-forming filamentous Cyanobacteria, Planktothrix agardhii (Gomont) Anagnostidis and Komárek 1988 (classified as MGF 5a and MBFG III) dominated Lake Lugano's phytoplankton almost all throughout the annual cycle and presented high densities for at least seven consecutive month-sampling dates. This species achieved extremely high values, peaking during early spring 2015 with values >62,000 ind ml−1. It maintained high densities during late autumn 2015, spring, and summer and only decayed in numbers and relative importance during autumn 2016.
The RDA performed with the two phytoplankton classification approaches (MFG and MBFG) revealed strong similar results (Fig. 7a and 7b). The distributions of functional groups were influenced by temporal fluctuations of physical and chemical conditions. The cumulative percentage of variance of the species–environment relationship explained by the first axis was 95.1% (p = 0.002) and 93.1% (p = 0.004), for MFG and MBFG, respectively. It was directly associated to DRP (r = 0.5001 and r = 0.5342) and TN (r = 0.4879 and r = 0.4887), and inversely to NH4 (r = −0.6273 and r = −0.6607) and Secchi transparency (r = −0.833 and r = −0.8337, MFG and MBFG, respectively, in all cases). The second axis explained 11.7 and 15.4% (MFG and MBFG, respectively) and was positively related to TP (r = 0.4538 and r = 0.4888, respectively). In both ordination analyses, samples were arranged according to a seasonal pattern. Samples of autumn 2015 were plotted in the upper side of the panel, related to high concentration of TP and characterized by the highest abundance of MFG 9b, MFG 3a, and MFG 7a (Fig. 7a) or MBFG IV, MBFG V, and MBFG VI (Fig. 7b). In turns, winter and spring 2015 samples were strongly influenced by the dominance of MFG 5a and MBGF III groups, situated in the right side of the panel when highest concentrations of TN and DRP, high turbidity conditions, and lowest water temperatures and NH4 values were recorded. Contrarily, summer and autumn 2016 samples showing contrasting environmental conditions were represented in the left side of the graphics and were mainly related to a more diversified community.
 |
Fig. 4 Phytoplanktonic chlorophyll a fluctuations during the annual cycle 2015–2016 in Lake Lugano. SP: samping point. |
 |
Fig. 5 Phytoplankton community attributes throughout the studied period in Lake Lugano, following the morpho-functional group (MFG) and the morphologically based functional group (MBFG) classifications. (a) Diversity and evenness for MFG. (b) Diversity and evenness for MBFG. (c) Similarity between sampling points considering both classification methods. SP: sampling point. |
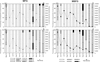 |
Fig. 6 Phytoplankton densities and proportions of the differing groups considering morpho-functional group (MFG) and the morphologically based functional group (MBFG) classifications in Lake Lugano for the studied period. SP: sampling point. |
 |
Fig. 7 Redundancy detrended analysis triplots based on the densities of (a) morpho-functional groups (MFG) proposed by Salmaso and Padisak (2007) and (b) morphologically based functional groups (MBFG) by Kruk et al. (2010). |
4 Discussion
Major problems associated with eutrophication of urban shallow lakes include water quality deterioration with increased nutrient loads, such as phosphorus and nitrogen, leading to high densities of pelagic algae (increased phytoplankton chlorophyll a concentrations), which diminish water transparency. The increased turbidity due to phytoplankton, and the often predominance of Cyanobacteria, decrease the recreational value of urban superficial waters for the society. Lake Lugano constitutes one case under this scenario. It is the largest shallow lake in Buenos Aires City, as evidenced by this and past monitoring studies (Rodríguez et al., 2003; Sinistro et al., 2004). During the annual cycle 2015–2016, water remained turbid almost all throughout the year. In eutrophic ecosystems, Secchi depth transparency is below 1 m (Qiu et al., 2001; Xiangcan, 2003; Chen et al., 2009) or even lower than 0.5 m. In Lake Lugano, the values for this parameter fall within this range coinciding with previous observations (Rodriguez et al. op. cit.; Sinistro et al. op. cit.). Being already reported as an hypertrophic water body more than 10 years ago, Lake Lugano's DRP and TP concentrations showed more than a three-fold rise since then, evidencing an increased trend toward water deterioration. The extremely high TN and NH4 concentrations also reveal the bad conditions of Lake Lugano and are similar or even higher than those encountered in highly impacted urban ponds and shallow lakes worldwide (Xiangcan, 2003; Segura et al., 2013; Yu et al., 2016; Rodríguez-Flores, 2017; Waajen, 2017). The increase in nutrient concentration can be the result of the input of runoff waters as this shallow lake functions as an overflow system. Waters flowing on the nearby impervious surfaces enter the lake through a channel, and also illegal cloacal discharges have been reported (Rodríguez et al., 2003). Urban population growth together with a limited investment in water supply and treatment infrastructure generate water quality problems (van der Bruggen et al., 2010) such as what we have observed in Lake Lugano. Another aspect closely related to water deterioration in freshwater ecosystems is dissolved oxygen concentration. On some occasions, this parameter remained well below (<5 mg L−1) the guideline values for the maintenance of aquatic biota (Cuenca del Plata, 1987), confirming the poor water quality of this urban shallow lake. In hand with what was observed for transparency and nutrients, Lake Lugano's phytoplankton chlorophyll a concentrations were, in general, typical of eutrophic systems and exceeded 100 μg L−1, evidencing its hypertrophic condition. The encountered values are within the ranges reported for other urban shallow lakes of differing latitudes (Fabre et al., 2010; Segura et al., 2013; Waajen et al., 2014; Araújo et al., 2016) and water bodies from Buenos Aires City (Avigliano et al., 2014; Rodríguez-Flores, 2017). The sole exception to these eutrophic/hypertrophic values was recorded during the cold season 2016, associated with the collapse of the P. agardhii bloom evidenced in the decrease in MFG 5a/MBFG III densities.
The importance of assessing the ecosystems health (Meyer, 1997) includes not only ecological and functional aspects but also societal needs. Water systems deteriorate at high rates in megapolis and thus the development of procedures for diagnosing the ecological status and the monitoring urban freshwater ecosystems should be emphasized. The study of the aquatic biota represents an effective method to assess the integrity and health status of freshwater ecosystems, and it is now well known that biomonitoring is more advantageous than chemical monitoring (Lyche-Solheim et al., 2013, and cites therein). Different biological components can be evaluated in this respect and lately, phytoplankton has been proposed for the assessment of the ecological status of freshwater ecosystems (Lepistö et al., 2004; Padisák et al., 2006; Lyche-Solheim et al., 2013). In deteriorated shallow lakes, phytoplankton density increases, leading to diversity decline (Xiangcan, 2003). In Lake Lugano, biodiversity and evenness assessed following both functional groups approaches showed generally low values with an even community structure only occasionally. The lowest values characterized moments when MFG 5a/MBFG III were dominant; as already stated these groups include a bloom-forming Cyanobacterium (P. agardhii). Reduced diversity is characteristic of deteriorated systems and can be associated with the presence of Cyanobacteria. This aspect has been highlighted in particular for eutrophic/hypertrophic urban shallow lakes and ponds where Planktothrix spp. is a commonly reported case example (Scasso et al., 2001; Crossetti et al., 2008, Toporowska and Pawlik-Skowrońska, 2014).
The functional group approach was used in distinctive fields of ecological research. In particular for phytoplankton, the applicability of the morpho-functional group approach (Salmaso and Padisak, 2007) in the derivation of water quality indexes has been recently assessed (Salmaso et al., 2015 and references therein). The criteria to define MFG are among the strongest traits for predicting the best competitors under different environmental conditions. In agreement with this, our results evidenced that the dynamics of MFG responded to temporal fluctuations of both physical and chemical conditions as evidenced by the high variance explained in the species–environment relationship by the multivariate analysis. During the study, Cyanobacteria in MFG 5a (filamentous algae Oscillatoriales), green-algae in MFG 9b (small <30 μm unicellular Chlorococcales), and in MFG 3a (autotrophic flagellates − Phytomonadina), and diatoms in MFG 7a (small <30 μm − centric diatoms) were the groups more abundantly represented in Lake Lugano's phytoplankton. The RDA revealed that MFG 5a was the functional group associated with high concentrations of TN and DRP. Represented by oscillatorian Cyanobacteria, the dominance of this group is generally related to the high availability of nutrients (Waajen, 2017). These water characteristics remained almost all throughout the study period. Eutrophic and hypertrophic lakes are susceptible to bloom formation and seasonal fluctuations in phytoplankton communities are commonly reduced (Xiangcan, 2003; Allende et al., 2009; Toporowska and Pawlik-Skowrońska, 2014; Waajen, 2017). The almost persistent year-round bloom of P. agardhii (main representative of MFG 5a) increased the turbidity of the water column. This condition negatively affects the development of green-algae, which lack motility (Happey-Wood, 1988). In this respect, the small <30 μm unicellular Chlorococcales (MFG 9b) only became relatively abundant when MFG 5a was not dominant. Nonfilamentous chlorococcalean colonies in MFG 11a (naked colonies) and in MFG 11b (gelatinous colonies) only “appeared” (in spite of their relative low numbers) when the bloom collapsed and transparency increased. When transparency was low, only flagellated green-algae were able to codominate the phytoplankton community, represented by unicellular autotrophic Phytomonadina (MFG 3a). The presence of flagella is an advantageous trait under this condition, promoting their growth in combination with high nutrient concentrations. MFG 3a was also related to high turbidity values in eutrophic shallow lakes subjected to agricultural impact shallow lakes (Izaguirre et al., 2012). Diatoms are susceptible to suffer sinking losses in stratified water columns due to their heavy siliceous walls, which distinguish them from other organisms (Padisák et al., 2003). In Lake Lugano, small and centric diatoms in MFG 7a were only relatively abundant on autumn 2015, probably due to increased mixing conditions that allowed their maintenance in the water column. The lack of rooted macrophytes in shallow lakes makes them susceptible to wind action (Scheffer et al., 1993) what guarantees the mixing of the water column and the concomitant sediment resuspension increasing the turbidity of the water column. This turbid environment provides adequate conditions for the development of algae adapted to cope with light constrains such as Planktothrix and can lead to blooms provided the nutrient requirements are fulfilled. As stated by Reynolds et al. (2002) in their trait-based functional classification of the freshwater phytoplankton, Planktothrix agardhii is a typical representative of the codon S1, characteristic of turbid mixed layers, presenting a high tolerance to light-deficient conditions. This scenario has been present in Lake Lugano and P. agardhii (MFG 5a; MBFG III) was the dominant species (and corresponding morpho-functional group) almost all throughout the annual cycle. In this respect, we can argue that under the Alternative Steady States framework (Scheffer et al., 1993), we can further recognize a phytoplankton turbid state dominated by low-light adapted Cyanobacteria, such as P. agardhii, when mixed and highly turbid conditions (provoked by increased sediment resuspension and not only due to increased algal densities) are met. The similarity encountered between the two sampling points both regarding the physical and chemical parameters and the structure and dynamics of the phytoplankton community provide further evidence of the characteristic mixed water of this shallow lake lacking associated vegetation during the 2015–2016 annual cycle. In this respect, we did not register the copious presence of palustrine, submerged and/or floating macrophytes throughout the study as reported previously for Lake Lugano (Rodríguez et al., 2003). Note that those authors found that in the presence of macrophytes it was the cyanobacterium Microsystis aeruginosa, representative of codon M (Reynolds et al., 2002), the typical bloom-former in Lake Lugano. Thus, in the previous study, more stagnant waters in eutrophic Lake Lugano might have provided the conditions required by this algae to show an exponential growth. In this respect, the dominance of morpho-functional group of Cyanobacteria under eutrophic conditions might depend on the stability of water column. Whereas P. agardhii (codon S1, MFG 5a/MBFG III) blooms are reported for mixed situations, Microsystis sp. (codon M, MFG 5c/MBFG III) requires more stagnant conditions (Reynolds et al., 2002; Padisák et al., 2009).
Interestingly, similar patterns in the seasonal development of the phytoplankton community as assessed considering the morphology-based functional group classification was encountered, and groups responded to the same set of physical and chemical variables as the morpho-functional groups. MBFG III (filaments of Nostocales and Oscillatoriales with aerotopes), MBFG V (unicellular flagellates of medium to large size), MBFG IV (organisms of medium size lacking specialized structures), MBFG VI (nonflagellated organisms with siliceous exoskeletons), and MBFG VII (large mucilaginous colonies) represented the phytoplankton community at different moments during the annual cycle. Again, the dominance and seasonal dynamic of the potentially toxic bloom-forming P. agardhii was detected by this classification method, representing the MBFG III. This group was also associated to high DRP and TN concentrations and low transparency. The close relationship between MBFG III and TN has been already reported for shallow lakes in highly human impacted areas (Izaguirre et al., 2012) and its dominance was reported to be associated to low light conditions and high TN values (Kruk and Segura, 2012). The success of Oscillatoriales in this morpho-functional group is in agreement with studies eutrophic shallow lakes (Bonilla et al., 2012; Waajen et al., 2014) and confirms that they can succeed in turbid, eutrophic lakes as originally proposed for Oscillatoriales (Scheffer et al., 1997). Studies on phytoplankton succession based on the MBFG approach revealed that the dominance of highly specialized groups, such as MBFG III, is expected to continue under stable conditions (Segura et al., 2013). The other groups only evidenced limited representativeness occasionally, and responded to the same environmental variables as the morpho-functional group classification. They were associated to high TP concentrations and became relatively important following the same pattern as described for the MFG approach, mostly when MBFG III (represented as MFG 5a) was not dominant.
Several studies have evaluated the deficiencies and the benefits of the functional group approach comparing, among others, MFG and MBFG in different types of freshwater ecosystems (Litchman et al., 2010; Izaguirre et al., 2012; Abonyi et al., 2014; Bortolini et al., 2014; Salmaso et al., 2015; Rodríguez-Flores, 2017). Some authors have stated that whereas the MFG approach can explain phytoplankton dynamics in relation to environmental variables (regional and temporal scales), the MBFG can better explain large-scale variations (Salmaso et al., 2015 and cites therein). The strength in the assessment of phytoplankton dynamics by the MFG (based on 33 groups) resides in the fact that it can explain changes in the phytoplankton in response to major environmental drivers representing an adequate tool to monitor this community. However, expertise in taxonomy is required at least up to the genera/order level. In turn, the application of MBFG classification (based on seven groups) is beneficial due to the relative simplicity and independence of taxonomic affiliation (Izaguirre et al., 2012), and diminishes the high dimensionality typical of phytoplankton community (Segura et al., 2013). The obvious disadvantage of MBFG with respect to the MFG is that the simplification in a reduced number of groups does not allow for a more detailed description of the phytoplankton assemblage composition. In this sense, MBFG is not able to detect a number of aspects that might be relevant when studying the phytoplankton assemblage (i.e., mixotrophic and autotrophic flagellates; chlorococcalean and chroococalean mucilagenous colonies). We coincide that, as stated by Kruk et al. (2010), the MBFG approach is coarse in comparison to other methods of phytoplankton classification (i.e., morpho-functional group by Salmaso and Padisak, 2007). However, its relative simplicity turns it to be an easy-to-use tool for determining the phytoplankton assemblage in urban shallow lakes. In our study, these two phytoplankton classifications produced similar and overlapping results as evidenced by more than one parameter (diversity, evenness) and by the multivariate analyses. Even though there was greater functional group diversity when considering MFG (based in a greater number of possible groups), dominance, codominance, and dynamics patterns were very similar for both classifications. This provides evidence that the MBFG was sensitive enough to characterize the phytoplankton community of Lake Lugano.
Some considerations regarding the prevalence of P. agardhii represented in MFG 5a/MBFG III are worth mentioning. This group achieved very high densities in spite of the low water temperatures in the cold season 2015. Thus, in Lake Lugano, a decrease in water temperature might not necessarily result in Cyanobacterial density decay as it was also reported for other small urban aquatic ecosystems (Fabre et al., 2010) at similar latitudes. Moreover, the absence of macrophytes in eutrophic systems favors the development of Cyanobacterial blooms (Allende et al., 2009). These aspects constitute a matter of concern, as year-round blooms can persist in Lake Lugano with the evident impact in their recreational value. As well, a potential risk for aquatic biota and humans derives from the cyanobacterial toxic blooms (Marie et al., 2012) and can result in fish kills. This issue has been already reported for Lake Lugano in 2003 when Microcystis aeruginosa (Kützing) Kützing reached densities that exceeded 100,000 ind ml−1 (Rodríguez et al., 2003). Common Cyanobacteria genera are known as toxin producers (i.e., Planktothrix (MFG 5a/MBGF III), Nostoc (MFG 5e/MBGF III), Dolychospernum Syn. Anabaena (MFG 5e/MBGF III), and Microcystis (MFG 5c/MBGF VII) and are repeatedly reported in urban aquatic ecosystems (Scasso et al., 2001; Rodríguez et al., 2003; Lv et al., 2011; Toporowska and Pawlik-Skowrońska, 2014; Waajen, 2017). Throughout our study, bloom-densities were registered for the filamentous Cyanobacteria P. agardhii. Even though we did not measure cyanobacterial-derived toxins, the potential toxicity of Planktothrix is recognized by different authors (Koramek and Komarková, 2004; Tonk et al., 2005; Hulot et al., 2012; Marie et al., 2012). We did not register fish mortality during our year-round study but the repeated presence of P. agardhii should not be disregarded. Thus, the method employed for the study of the phytoplankton of these systems should include the possibility to identify and follow-up the dynamics of these potentially toxic algae. It is worth mentioning that the phytoplankton classification methods employed in our study were able to detect both the dominance and the dynamics of P. agardhii (MFG 5a/MBFG III).
5 Conclusion
The applicability of the MFG or the MBFG approach depends on the type of research being held and the aim of the study. Our results showed that the dynamics of the phytoplankton communities as assessed by MFG (Salmaso and Padisak, 2007) and MBFG (Kruk et al., 2010) classifications similarly described their attributes and followed overlapping patterns of fluctuation responding to changes in environmental conditions in Lake Lugano. The two methods were sensitive enough to detect the presence of potentially toxic filamentous Cyanobacteria, as well as bloom episodes and their collapses. As the MFG classification requires more specialized skills in order to classify the organisms encountered than the MBFG, we consider that MBFG classification was an adequate, easy-to-handle method for monitoring Lake Lugano on an annual basis. Due to the similarity in the results obtained, the latter approach proved to be sensitive enough and could represent an objective way to study the phytoplankton dynamics by water quality managers. However, expert judgment is also required when alert signals are detected in the monitored ecosystem.
The application of the MBFG classification for biomonitoring purposes of urban shallow lakes and ponds should necessarily be done in combination with the identification of the dominant species and/or potentially toxic bloom-forming species. In particular, changes in MBFG III and MBFG VII should be closely followed as different highly toxic genera are classified in these groups. As well, further studies should include the estimation of cyanobacterial-derived toxin concentrations in water as a prerequisite to evaluate its quality in relation to the recreational value. We also consider that the applicability of the MBFG approach deserves to be further explored as a promising tool for the biomonitoring of different types of urban water bodies located in recreational parks, ecological reserves, and even in domestic ponds.
Acknowledgments
This research has been supported by the Agencia Nacional de Promoción Científica y Tecnológica (Argentina) (BID − PICT 2013–2323). Authors wish to thank Agencia de Protección Ambiental (CABA-Argentina) and Corporación Buenos Aires Sur. We are grateful to two reviewers of a previous version of the manuscript for their useful comments and suggestions.
References
- Abonyi A, Leitão M, Stanković I, Borics G, Várbíró G, Padisák J. 2014. A large river (River Loire, France) survey to compare phytoplankton functional approaches: do they display river zones in similar ways? Ecol Indic 46: 11–22. [Google Scholar]
- Allende L, Tell G, Zagarese HE, Torremorell A, Pérez G, Bustingorry J, Escaray R, Izaguirre I. 2009. Phytoplankton and primary production in clear-vegetated, inorganic-turbid and algal-turbid shallow lakes from Pampa Plain (Argentina). Hydrobiologia 624: 45–60. [Google Scholar]
- APHA (American Public Health Association). 2005. Standard Methods for the Examination of Water and Wastewaters. Washington: APHA. [Google Scholar]
- Araújo F, Becker V, Attayde AL. 2016. Shallow lake restoration and water quality management by the combined effects of polyaluminium chloride addition and benthivorous fish removal: a field mesocosm experiment. Hydrobiologia 778: 243–252. [Google Scholar]
- Avigliano L, Vinocur A, Chaparro G, Tell G, Allende L. 2014. Influence of re-flooding on phytoplankton assemblages in a temperate wetland following prolonged drought. J Limnol 73: 247–262. [Google Scholar]
- Becklioglu M, Meerhoff M, Søndergaard M, Jeppesen E. 2011. Eutrophication and restoration of shallow lakes from a cold temperate to a warm Mediterranean and a (sub)tropical climate. In: Ansari AA, Lanza GR, Gill SS, Rast W, eds. Eutrophication: Causes, Consequences and Control. The Netherlands: Springer, pp. 91–108. [Google Scholar]
- Benincá E, Huisman J, Heerkloss R, Jöhknl KD, Branco P, Van Nes EH, Scheffer M, Ellner SP. 2008. Chaos in a long-term experiment with a plankton community. Nature 451: 822–825. [CrossRef] [PubMed] [Google Scholar]
- Blaum N, Mosner E, Schwager M, Jeltsch F. 2011. How functional is functional? Ecological groupings in terrestrial animal ecology: towards an animal functional type approach. Biodivers Conserv 20: 2333–2345. [Google Scholar]
- Bonilla S, Aubriot L, Soares MCS, González-Piana M, Fabre A, Huszar VLM, Lürling M, Antoniades D, Padisák J, Kruk C. 2012. What drives the distribution of the bloom-forming cyanobacteria Planktothrix agardhii and Cylindrospermopsis raciborskii? FEMS Microbiol Ecol 79: 594–607. [CrossRef] [PubMed] [Google Scholar]
- Bortolini JC, Rodrigues LC, Jatyi S, Train S. 2014. Phytoplankton functional and morphological groups as indicators of environmental variability in a lateral channel of the Upper Paraná River floodplain. Acta Limnol Bras 26: 98–108. [CrossRef] [Google Scholar]
- Carpenter SR, Stanly EH, Van der Zanden MJ. 2011. State of the world's freshwater ecosystems: physical, chemical, and biological changes. Annu Rev Environ Resour 36: 75–99. [Google Scholar]
- Chen K-N, Bao C-H, Zhou W-P. 2009. Ecological restoration in eutrophic Lake Wuli: a large enclosure experiment. Ecol Eng 35: 1646–1655. [Google Scholar]
- Crossetti LO, Bicudo DC, Bicudo CEM, Bini LM. 2008. Phytoplankton biodiversity changes in a shallow tropical reservoir during the hypertrophication process. Braz J Biol 68: 1061–1067. [Google Scholar]
- Cuenca del Plata. 1987. Evaluación de la calidad de las aguas y control de la contaminación. Segunda Reunión de Contrapartes Técnicas de los países de la Cuenca del Plata. Documento de Trabajo 021. [Google Scholar]
- DGEC (Dirección Nacional de Estadísticas y Censo). 2016. Gobierno de la Ciudad de Buenos Aires. Departamento de Cartografía. https://www.estadisticaciudad.gob.ar/eyc/wp-content/uploads/2016/08/DP051603.pdf [Google Scholar]
- Fabre A, Carballo C, Hernández E, Piriz P, Bergamino L, Mello L, González S, Pérez G, León JG, Aubriot L, Bonilla S, Kruk C. 2010. El nitrógeno y la relación zona eufótica/zona de mezcla explican la presencia de cianobacterias en pequeños lagos subtropicales, artificiales de Uruguay. Panam J Aquat Sci 5: 112–125. [Google Scholar]
- Fazio A, O'farrell I. 2005. Phytoplankton and water quality in a shallow lake: a response to secondary salinization (Argentina). Wetlands 25: 531–541. [CrossRef] [Google Scholar]
- Francis RA, Chadwick MA. 2012. What makes a species synurbic? Appl Geogr 32: 514–521. [Google Scholar]
- Gledhill DG, James P, Davies DH. 2008. Pond density as a determinant of aquatic species richness in an urban landscape. Landsc Ecol 23: 1219–1230. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electronica 4: 9. [Google Scholar]
- Happey-Wood C. 1988. Ecology of freshwater planktonic green algae. In: Sandgren CD, ed. Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge: Cambridge University Press, pp. 175–226. [Google Scholar]
- Henny C, Meutia AA. 2014. Urban lakes in Megacity Jakarta: risk and management plan for future sustainability. Procedia Environ Sci 20: 737–746. [Google Scholar]
- Hirose M, Nishibe Y, Ueki M, Nakano S-H. 2003. Seasonal changes in the abundance of autotrophic picoplankton and some environmental factors in hypereutrophic Furuike Pond. Aquat Ecol 37: 37–43. [Google Scholar]
- Hulot FD, Carmignac D, Legendre S, Yéprémian C, Bernards C. 2012. Effects of microcystin-producing and microcystin-free strains of Planktothrix agardhii on long-term population dynamics of Daphnia magna . Ann Limnol − Int J Lim 48: 337–347. [CrossRef] [Google Scholar]
- Izaguirre I, Allende L, Escaray R, Bustingorry J, Pérez G, Tell G. 2012. Comparison of morpho-functional phytoplankton classifications in human-impacted shallow lakes with different stable states. Hydrobiologia 698: 203–216. [Google Scholar]
- Jovanovic J, Trbojevic I, Subakov Simic G, Popovic S, Predojevic D, Blagojevic A, Karadžic V. 2017. The effect of meteorological and chemical parameters on summer phytoplankton assemblages in an urban recreational lake. Knowl Manag Aquat Ecosyst 418: 48. [CrossRef] [Google Scholar]
- Kamenir Y, Dubinsky Z, Zohary T. 2004. Phytoplankton size structure stability in a meso-eutrophic subtropical lake. Hydrobiologia 520: 89–104. [Google Scholar]
- Kamenir Y, Dubinsky Z, Zohary T. 2006. The long-term patterns of phytoplankton taxonomic size-structure and their sensitivity to perturbation: a Lake Kinneret case study. Aquat Sci 68: 490–501. [Google Scholar]
- Kruk C, Segura AM. 2012. The habitat template of phytoplankton morphology-based functional groups. Hydrobiologia 698: 191–202. [Google Scholar]
- Kruk C, Huszar VLM, Peeters ETHM, Bonilla S, Costa L, Lürling M, Reynolds CS, Scheffer M. 2010. A morphological classification capturing functional variation in phytoplankton. Freshw Biol 55: 614–627. [Google Scholar]
- Kruk C, Peeters ETHM, Van Nes EH, Huszar VLM, Costa LS, Scheffer M. 2011. Phytoplankton community composition can be predicted best in terms of morphological groups. Limnol Oceanogr 56: 110–118. [Google Scholar]
- Lepistö L, Holopainen A-L, Vuoristo H. 2004. Type-specific and indicator taxa of phytoplankton as a quality criterion for assessing the ecological status of Finnish boreal lakes. Limnologica 34: 236–248. [Google Scholar]
- Litchman E, De Tezanos Pinto P, Klausmeier CA, Thomas MK, Yohiyama K. 2010. Linking traits to species diversity and community structure in phytoplankton. Hydrobiologia 653: 15–28. [Google Scholar]
- Lv J, Wu H, Chen M. 2011. Effects of nitrogen and phosphorus on phytoplankton composition and biomass in 15 subtropical, urban shallow lakes in Wuhan, China. Limnoligica 41: 48–56. [CrossRef] [Google Scholar]
- Lyche-Solheim A, Feld CK, Birk S, Phillips G, Carvalho L, Morabito G, Mischke U, Willby N, Søndergaard M, Hellsten S, Lolada A, Mjelde M, Böhmer J, Miler O, Pusch MT, Argillier C, Jeppesen E, Lauridsen TL, Poikane S. 2013. Ecological status assessment of European lakes: a comparison of metrics for phytoplankton, macrophytes, benthic invertebrates and fish. Hydrobiologia 407: 57–74. [Google Scholar]
- MacGregor-Fors I. 2011. Misconceptions or misunderstandings? On the standardization of basic terms and definitions in urban ecology. Landsc Urban Plan 100: 347–349. [Google Scholar]
- Margalef R. 1978. Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol Acta 1: 493–509. [Google Scholar]
- Marie B, Huet H, Marie A, Djediat C, Pusieux-Dao A, Trinchet C, Edery M. 2012. Effects of a toxic cyanobacterial bloom (Planktothrix agardhii) on fish: insights from istopathological and quantitative proteomic assessments following the oral exposure of medaka fish (Oryzias latipes). Aquat Toxicol 114: 39–48. [CrossRef] [PubMed] [Google Scholar]
- Marker AFH, Nusch A, Rai H, Riemann B. 1980. The measurement of photosynthetic pigments in freshwater and standardization of methods: conclusions and recommendations. Arch Hydrobiol Beih Ergebn Limnol 14: 91–106. [Google Scholar]
- Meerhoff M, Mazzeo N, Moss B, Rodríguez-Gallego L. 2003. The structuring role of free-floating versus submerged plants in a subtropical shallow lake. Aquat Ecol 37: 377–391. [Google Scholar]
- Meyer JL. 1997. Stream health: incorporating the human dimension to advance stream ecology. J N Am Benthol Soc 16: 439–447. [CrossRef] [Google Scholar]
- Naselli-Flores L, Barone R. 2011. Fight on plankton! Or, phytoplankton shape and size as adaptive tools to get ahead in the struggle for life. Cryptogam Algol 32: 157–204. [CrossRef] [Google Scholar]
- Olding D, Hellebust A, Douglas M. 2000. Phytoplankton community composition in relation to the water quality and water-body morphometry in urban lakes, reservoirs, and ponds. Can J Fish Aquat Sci 57: 2163–2174. [Google Scholar]
- Padisák J, Soróczki-Pintér E, Rezner Z. 2003. Sinking properties of some phytoplankton shapes and the relation of form resistance to morphological diversity plankton: an experimental study. Hydrobiologia 500: 243–257. [Google Scholar]
- Padisák J, Grigorszky I, Borics G, Soróczki-Pintér E. 2006. Use of phytoplankton assemblages for monitoring ecological status of lakes within the Water Framework Directive: the assemblage index. Hydrobiologia 553: 1–14. [Google Scholar]
- Padisák J, Crossetti LO, Naselli-Flores L. 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19. [Google Scholar]
- Pickett STA, Cadenasso ML. 2006. Advancing urban ecological studies: frameworks, concepts, and results from the Baltimore ecosystem study. Austral Ecol 31: 114–125. [Google Scholar]
- Platt T, Denman K. 1978. The Structure of Pelagic Ecosystem. Rapports et Proces-Verbaux des Reunions, Conseil Permanent International pour l'Exploration de la Mer, 173: 60–65. [Google Scholar]
- Qiu D, Zhenbin W, Baoyuan L, Jiaqi D, Guiping F, Feng H. 2001. The restoration of aquatic macrophytes for improving water quality in a hypertrophic shallow lake in Hubei Province, China. Ecol Eng 18: 147–156. [Google Scholar]
- Reynolds CS. 1980. Phytoplankton assemblages and their periodicity in stratifying lake systems. Holarctic Ecol 3: 141–159. [Google Scholar]
- Reynolds CS. 1988a. Functional morphology and the adaptive strategies of freshwater phytoplankton. In: Sandgren CD, ed. Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge: Cambridge University Press, pp. 388–433. [Google Scholar]
- Reynolds CS. 1988b. The concept of ecological succession applied to seasonal periodicity of freshwater phytoplankton. Verh Internat Verein Limnol 23: 683–691. [Google Scholar]
- Reynolds CS. 2006. The Ecology of Phytoplankton. Cambridge: Cambridge University Press. [Google Scholar]
- Reynolds CS, Huszar V, Kruk C, Naselli-Flores L, Melo S. 2002. Towards a functional classification of the freshwater phytoplankton. J Plankton Res 24: 417–428. [Google Scholar]
- Rodríguez-Flores C. 2017. Estructura y dinámica del fitoplancton de tres estanques urbanos de la ciudad de Buenos Aires: análisis de floraciones en época estival (2014–2015). Tesis Magìster Universidad de Buenos Aires. [Google Scholar]
- Rodríguez P, De Tezanos Pinto P, Sinistro S, Pizarro H. 2003. Estudio del estado trófico del lago Lugano (Parque Roca, ciudad de Buenos Aires) Primer informe de avance − 21 de Abril al 22 de julio de 2003. Control De Lagos De La Ciudad De Buenos Aires. Dirección General de Política y Evaluación Ambiental Gobierno de la Ciudad Autónoma de Buenos Aires Departamento de Ecología, Genética y Evolución Facultad de Ciencias Exactas y Naturales Universidad de Buenos Aires, 25. [Google Scholar]
- Salmaso N, Padisak J. 2007. Morpho-functional groups and phytoplankton development in two deep lakes (Lake Garda, Italy and Lake Stechlin, Germany). Hydrobiologia 578: 97–112. [Google Scholar]
- Salmaso N, Naselli-Flores L, Padisák J. 2015. Functional classifications and their application in phytoplankton ecology. Freshw Biol 60: 603–619. [Google Scholar]
- Scasso F, Mazzeo N, Gorga J, Kruk C, Lacerot G, Clemente J, Fabián D, Bonilla S. 2001. Limnological changes in a sub-tropical shallow hypertrophic lake during its restoration: two years of a whole-lake experiment. Aquat Conserv 11: 31–44. [Google Scholar]
- Scheffer M, Hosper SH, Meijer ML, Moss B, Jeppesen E. 1993. Alternative equilibria in shallow lakes. Trends Ecol Evol 8: 275–279. [CrossRef] [PubMed] [Google Scholar]
- Scheffer M, Rinaldi S, Gragnani A, Mur L, Van Nes EH. 1997. On the dominance of filamentous Cyanobacteria in shallow, turbid lakes. Ecology 78: 272–282. [Google Scholar]
- Segura AM, Kruk C, Calliari D, Fort H. 2013. Use of a morphology-based functional approach to model phytoplankton community succession in a shallow subtropical lake. Freshw Biol 58: 504–512. [Google Scholar]
- Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. 2006. From patterns to emerging processes in mechanistic urban ecology. TRENDS Ecol Evol 21: 186–191. [CrossRef] [PubMed] [Google Scholar]
- Sinistro R, Rodríguez P, De Tezanos Pinto P, Pizarro H. 2004. Estudio del estado trófico del lago Lugano (Parque Roca, Ciudad de Buenos Aires). Informe Técnico Final − 1 de Abril al 31 de Diciembre de 2003. Control de Lagos de la Ciudad de Buenos Aires. Dirección General de Política y Evaluación Ambiental. Gobierno de la Ciudad Autónoma de Buenos Aires. Departamento de Ecología, Genética y Evolución Facultad de Ciencias Exactas y Naturales Universidad de Buenos Aires. [Google Scholar]
- Stanca E, Cellamare M, Basset A. 2013. Geometric shape as a trait to study phytoplankton distributions in aquatic ecosystems. Hydrobiologia 701: 99–116. [Google Scholar]
- Taranu ZE, Gregory-Eaves I, Leavitt PR, Bunting L, Buchaca T, Catalan J, Domaizon I, Guilizzoni P, Lami A, Mcgowan S, Moorhouse H, Morabito G, Pick FR, Stevenson MA, Thompson PL, Vinebrooke RD. 2015. Acceleration of cyanobacterial dominance in north temperate-subarctic lakes during the Anthropocene. Ecol Lett 18: 375–384. [Google Scholar]
- ter Braak CJF, Verdonschot PFM. 1995. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci 57: 255–289. [Google Scholar]
- Tolotti M, Thies H, Nickus U, Psenner R. 2012. Temperature modulated effects of nutrients on phytoplankton changes in a mountain lake. Hydrobiologia 698: 61–75. [Google Scholar]
- Tonk L, Visser PM, Christiansen G, Dittmann E, Snelder EOFM, Wiedner C, Mur LR, Huisman J. 2005. The microcystin composition of the Cyanobacterium Planktothrix agardhii changes toward a more toxic variant with increasing light intensity. Appl Environ Microbiol 71: 5177–5181. [Google Scholar]
- Toporowska M, Pawlik-Skowrońska B. 2014. Four-year study on phytoplankton biodiversity in a small hypertrophic lake affected by water blooms of toxigenic cyanobacteria. Pol J Environ Stud 23: 491–499. [Google Scholar]
- Ütermöhl H. 1958. Zur Vervollkommnung der quantitative Phytoplankton Methodik. Mitt Internat Ver Limnol 9: 1–38. [Google Scholar]
- Valderrama JC. 1981. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Mar Chem 10: 109–122. [Google Scholar]
- van der Bruggen B, Borghgraef K, Vinckier C. 2010. Causes of water supply problems in urbanised regions in developing countries. Water Resour Manag 24: 1885–902. [CrossRef] [Google Scholar]
- Waajen GWAM. 2017. Eco-engineering for clarity: clearing blue-green ponds and lakes in an urbanized area. PhD thesis, Wageningen University, The Netherlands, 308 p. [Google Scholar]
- Waajen GWAM, Faasen EJ, Lürling M. 2014. Eutrophic urban ponds suffer from cyanobacterial blooms: Dutch examples. Environ Sci Pollut Res 21: 9983–9994. [CrossRef] [Google Scholar]
- Weithoff G. 2003. The concepts of “plant functional types” and “functional diversity” in lake phytoplankton: a new understanding of phytoplankton ecology? Freshw Biol 48: 1669–1675. [Google Scholar]
- WWAP (World Water Assessment Programme). 2012. The United Nations World Water Development Report 4: Managing Water under Uncertainty and Risk. UNESCO, Paris. [Google Scholar]
- Xiangcan J. 2003. Analysis of eutrophication state and trend for lakes in China. J Limnol 62: 60–66. [Google Scholar]
- Yu J, Liu Z, Li K, Chen F, Guan B, Hu Y, Zhong P, Tang Y, Zhao X, He H, Zeng H, Jeppesen E. 2016. Restoration of shallow lakes in subtropical and tropical China: response of nutrients and water clarity to biomanipulation by fish removal and submerged plant transplantation. Water 8: 438–450. [Google Scholar]
- Zar JH. 1999. Biostatistical Analysis, 4th ed. New Jersey: Prentice Hall. [Google Scholar]
Cite this article as: Allende L, Fontanarrosa MS, Murno A, Sinistro R. 2019. Phytoplankton functional group classifications as a tool for biomonitoring shallow lakes: a case study. Knowl. Manag. Aquat. Ecosyst., 420, 5.
All Tables
Ranges encountered for physical and chemical parameters in Lake Lugano throughout the studied period 2015–2016 (SD: standard deviation, min: minimum, max: maximum). Turbid: turbidity, NH4: ammonia, N-NO3: nitrates, TN: total nitrogen, DRP: dissolved reactive phosphorus, TP: total phosphorus, Cond.: conductivity, Water Temp.: water temperature, DO: dissolved oxygen, Secchi: Secchi transparency, Chlor a: phytoplanctonic chlorophyll a concentration, SP: sampling point.
All Figures
 |
Fig. 1 Location of Lake Lugano in Parque Roca (Buenos Aires, Argentina), and the respective sampling points (SP1 and SP2). |
| In the text | |
 |
Fig. 2 Environmental variables measured in Lake Lugano during the annual cycle 2015–2016. (a) Water temperature. (b) Conductivity. (c) pH. (d) Dissolved oxygen concentration. (e) Transparency. SP: sampling point. |
| In the text | |
 |
Fig. 3 Fluctuations in nutrient concentrations throughout the studied period in Lake Lugano. (a) NH4: ammonia, (b) N-NO3: nitrates, (c) DRP: dissolved reactive phosphorus, (d) TP: total phosphorus, (e) TN: total nitrogen. SP: sampling point. |
| In the text | |
 |
Fig. 4 Phytoplanktonic chlorophyll a fluctuations during the annual cycle 2015–2016 in Lake Lugano. SP: samping point. |
| In the text | |
 |
Fig. 5 Phytoplankton community attributes throughout the studied period in Lake Lugano, following the morpho-functional group (MFG) and the morphologically based functional group (MBFG) classifications. (a) Diversity and evenness for MFG. (b) Diversity and evenness for MBFG. (c) Similarity between sampling points considering both classification methods. SP: sampling point. |
| In the text | |
 |
Fig. 6 Phytoplankton densities and proportions of the differing groups considering morpho-functional group (MFG) and the morphologically based functional group (MBFG) classifications in Lake Lugano for the studied period. SP: sampling point. |
| In the text | |
 |
Fig. 7 Redundancy detrended analysis triplots based on the densities of (a) morpho-functional groups (MFG) proposed by Salmaso and Padisak (2007) and (b) morphologically based functional groups (MBFG) by Kruk et al. (2010). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


