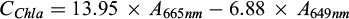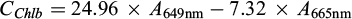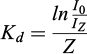| Issue |
Knowl. Manag. Aquat. Ecosyst.
Number 426, 2025
Riparian ecology and management
|
|
|---|---|---|
| Article Number | 16 | |
| Number of page(s) | 8 | |
| DOI | https://doi.org/10.1051/kmae/2025013 | |
| Published online | 03 June 2025 | |
Research Paper
Investigating tools for biomonitoring browning in streams: chlorophyll content of Fontinalis antipyretica
Ecosystems and Environment Research Programme, Faculty of Biological and Environmental Sciences, University of Helsinki, P.O. Box 65, FI-00014, Finland
* Corresponding author: salla.rajala@helsinki.fi
Received:
12
March
2025
Accepted:
5
May
2025
The chlorophyll content of the bryophyte species Fontinalis antipyretica was studied in seven small boreal streams of varying water quality. The aim was to determine whether the chlorophyll content of F. antipyretica responds to increasing water colour, and if the bryophyte could thus be used as a bioindicator for browning in stream environments. Significant variation in the CHL contents (CHL a+b, CHL a:b) of F. antipyretica was observed between streams of differing water colour. Despite this, no significant effect of water colour or the dissolved organic carbon (DOC) concentration on the CHL contents (CHL a+b, CHL a:b) was observed. However, the CHL a:b ratio decreased significantly with increasing light attenuation coefficient, thus suggesting that light is an important factor causing physiological changes in F. antipyretica. The DOC concentration explained one-third of the light attenuation coefficient, whilst no significant effect of water colour was found. Our findings suggest that whilst the CHL a:b of F. antipyretica can indicate changes in light conditions, it does not directly show a clear pressure-response association with increased water colour or DOC concentration.
Key words: Bioindicator / bryophyte / lotic waters / brownification / Water Framework Directive
© S. Rajala et al., Published by EDP Sciences 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
Highlights
CHL content of F. antipyretica varied between streams of varying water colour.
Variation in CHL content was traced to differences in light availability.
Water colour or DOC concentration did not affect CHL content of F. antipyretica.
CHL content of F. antipyretica is probably not suitable indicator for browning.
1 Introduction
Throughout the Northern Hemisphere an increase in water colour, a phenomenon called browning, has been observed in freshwaters during recent decades (Monteith et al., 2007; Weyhenmeyer et al., 2016). Browning is caused by increased loading of dissolved organic carbon (DOC) and iron (Fe) from the catchment (Monteith et al., 2007; Kritzberg and Ekström, 2012), and it is strongly driven by climate change-induced phenomena, such as increased precipitation and elevated temperature (Weyhenmeyer and Karlsson 2009; de Wit et al., 2016). The role of land-use activities, especially ditching and forestry operations, is also prominent (Estlander et al., 2021), whilst decreased atmospheric sulphate deposition also impacts browning due to the increased solubility of organic matter from the soils (Evans et al., 2012; Finstad et al., 2016).
Browning has several detrimental effects on aquatic ecosystems. It decreases light penetration in the water column and changes its spectral composition by increasing the absorption of short-wavelength light (Eloranta, 1999). The decrease in photosynthetically important short-wavelength light consequently decreases primary production, changing ecosystems from autotrophic to more heterotrophic (Thrane et al., 2014). Particularly in lakes, thermal stratification becomes more steep as the darker water absorbs more solar irradiance (Eloranta, 1999).
The increased DOC concentration decreases water pH, which can be especially harmful to vulnerable stream ecosystems (Petrin et al., 2007). Sedimentation rate increases as more organic matter is descending into streams from catchments, reducing macrophyte growth surfaces and burying deadwood, making the bottom an unsuitable habitat for many species (Kuglerová et al., 2021).
Although browning has several negative effects on both lake and stream ecosystems, it has not been sufficiently taken into account in water monitoring programmes (Sepp et al., 2018; Albrecht et al., 2024; Horppila et al., 2024). For example, the monitoring programmes of the European Water Framework Directive (WFD) have come under scrutiny because the biological parameters currently used in the WFD are mainly targeted for monitoring eutrophication and are not suitable for monitoring browning (Sepp et al., 2018; Horppila et al., 2024). The need for new bioindicators is urgent especially in streams, because their rapidly changing hydrology makes physical and chemical monitoring methods insufficient for reflecting long-term environmental changes (Li et al., 2010). Even though biological monitoring methods—such as the use of diatoms, zoobenthos, and fish—have been developed for streams and rivers for example in Finland, they face the same issue as those used in lakes: the indicators are primarily designed to monitor eutrophication (Sepp et al., 2018; Horppila et al., 2024).
Due to human activities, many small streams are also very disturbed ecosystems whose higher land-water interface make them more vulnerable to changes in catchment land-use than lakes (Tolkkinen et al., 2020; Kuglerová et al., 2021). Since disturbances in small streams also accumulate into downstream bodies of water (Asmala et al., 2019), no restoration methods can be done in these downstream areas without having to monitor the status of smaller streams (Gomi et al., 2002; Räike et al., 2016).
One of the important biological quality elements (BQEs) in the status assessment of waterbodies is macrophytes (Penning et al., 2008). In contrast to macrofauna, which can to some extent migrate to more suitable habitats, macrophytes are sessile organisms that cannot escape environmental stressors, making them particularly important as bioindicators of environmental change (Bhatta and Patra, 2020). In reference to tracking the effects of browning, one of the macrophyte-based bioindicators suggested is the chlorophyll a to b ratio (CHL a:b) of the floating-leaved macrophyte species yellow water-lily (Nuphar lutea (L.) Sm.) (Horppila et al., 2022; Rajala et al., 2024, 2025). The potential of this bioindicator arises from the adaptation mechanism of plants as primary producers to decreasing light (Kume et al., 2018). With decreasing short-wavelength light, a phenomenon that is specifically linked to browning, macrophytes adapt to changing light conditions by increasing their CHL b concentration in relation to CHL a concentration (Barko and Filbin, 1983; Horppila et al., 2022; Rajala et al., 2024).
In comparison to the macrophyte BQEs currently employed in the WFD, which rely on the macrophyte community composition analysis, utilizing a macrophyte physiology-based indicator would offer several advantages. Species community composition analyses can be vulnerable to methodological variation arising from biases in taxonomical identification (Lyche-Solheim et al., 2013). In addition, due to the spatial variability in macrophyte abundance, several sampling stations are needed for macrophyte community composition analysis (Lyche-Solheim et al., 2013). For the macrophyte physiology-based monitoring method, CHL a:b, the spatial variability within lakes is low, and the method expresses a consistent seasonal variation across lakes with differing water quality, thus enabling a uniform standardization of the sampling process (Rajala et al., 2024, 2025).
A species commonly used for stream biomonitoring is the bryophyte greater water-moss (Fontinalis antipyretica Hedw.) (Alaoui et al., 2021). Fontinalis antipyretica exhibits wide ecological tolerance, is widely distributed, long-lived, tolerant of pollution and has a high accumulation capacity, which is why it has been considered as a good bioindicator e.g. for metal pollution (Vuori and Muotka, 1999; Alaoui et al., 2021). Due to its excellent biomonitoring properties, it could also serve as a good indicator for browning. However, since F. antipyretica shows several physiological differences compared with vascular plants, the impact of increasing water colour on their CHL contents (CHL a+b, CHL a:b) remains obscure. Bryophytes lack conductive systems, which changes the way they regulate nutrients and light, as well as capture CO2 (Glime, 2014). In addition, F. antipyretica is a shade-adapted plant, which can also impact its responses to decreasing light (Proctor and Smirnoff, 2011). Therefore, the effects of changing light conditions on F. antipyretica were examined in this study. The aim of the study was to investigate, if the water colour of the stream effects chlorophyll content (CHL a+b/a:b) of F. antipyretica, and if the CHL content could thus be used as an indicator of browning in streams. The specific research questions were: 1) Does the CHL content (CHL a+b/ a:b) of F. antipyretica differ between streams? and 2) Does the water colour, DOC concentration or light attenuation coefficient (Kd) affect the CHL content (CHL a+b/ a:b) of F. antipyretica.
2 Materials and methods
2.1 Selected study sites
The study was conducted in July 2023 in seven small streams located near the Evo district (61° 13′N, 25° 12′E) (Fig. 1). Due to the limited knowledge on the ecological state of small streams, we selected the streams with catchment areas less than 100 km2. In order to study the effect of different water colour on the CHL content of F. antipyretica, we selected streams with as wide water colour range as possible. Sampling sites were chosen to ensure minimal shading of the overlying canopy and by selecting open areas. Other vegetation within the sampling areas was limited, and it was mainly composed of F. antipyretica and N. lutea, except in Heinäjoki, Mustajoki and Ormijoki, where N. lutea was absent in the sampling site. The wetted width at the sampling site varied between 1.3 m and 9 m, and all the streams were characterized with a coarse-grained bottom.
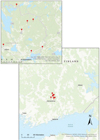 |
Fig. 1 Locations of the study lakes. |
2.2 Macrophyte sampling and analysis
Submerged macrophytes (F. antipyretica) were collected from the running water area of each stream. Three replicate samples were collected from a 10 cm water depth, placed in dark plastic bags, and stored in cool, dark conditions (+4 °C) until they were taken to the laboratory on the same day. In the laboratory, the F. antipyretica samples were washed with tap water to remove periphyton. For the CHL a+b analysis, 1 cm × 1.5 cm of phyllids were cut, wrapped in aluminium foil and stored in a freezer (–18 °C) until analysis. According to recent studies, freezing is the most adequate method to preserve CHL samples prior analysis (Villares et al., 2024). After freezing, the samples were macerated, and then weighted samples were extracted in 95% ethanol at 75 °C for 5 min, following ultrasound treatment (Horppila et al., 2022). The extract was also filtered using Whatman GF/C filters (pore size 1.2 μm), to remove particulate matter. The CHL a and b concentrations from the F. antipyretica samples were calculated with the equation of Lichtenthaler and Wellburn (1983):
where C is the chlorophyll μg ml−1 plant extract, and A is the measured absorbance at different wavelengths.
2.3 Physicochemical sampling
Water samples for water colour and DOC analyses were taken with a bottle sampler from the same location as the bryophyte samples. The water colour analysis was conducted with a Shimadzu UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) as the absorbance of light at a 410 nm wavelength. After the spectrophotometric analysis, the results were converted to mg Pt l−1, following the standard SFS-EN ISO 7887 (Finnish Standards Association, 2011). The DOC concentration was measured with a Shimadzu TOC 5000A analyser, following the standard SFS-EN 1484 (Finnish Standards Association, 1997).
In addition to the water samples, the water temperature and pH were measured with a YSI 6600 V2 sonde (YSI Inc., Yellow Springs, OH, USA), and the light intensity in the photosynthetically active radiation (PAR) wavelengths (400–700 nm) at the water column was measured with an LI-1500 data logger equipped with an LI-192SA Quantum Sensor (LI-COR Biosciences, Lincoln, NB, USA). The Kd was calculated with the equation:
where I0 and IZ are light intensities just below the surface and at depth Z (0.1 m), respectively (Scheffer, 1998).
2.4 Statistical analysis
The interstream differences in the water colour, DOC concentration and CHL a+b/a:b of F. antipyretica were studied with a one-way analysis of variance (ANOVA) and with Bonferroni t-tests. Prior to the ANOVA, the Shapiro–Wilk normality test and Levene's test for homogeneity of variance were computed. Based on these analyses, all data except for water colour were ln-transformed to improve normality.
Although DOC is usually the main determinant of water colour, the effects of DOC and water colour on light attenuation may differ as a result of Fe bound to organic substances (Horppila et al., 2023). Therefore, the effect of water colour, DOC concentration and Kd on the CHL a+b/a:b content of F. antipyretica was studied with a linear regression analysis. Additionally, linear regression analysis was used to investigate, whether the water colour and DOC concentration affect Kd in the streams. For the linear regression analysis, all data were ln-transformed prior analysis. All data analyses were computed with RStudio version 4.4.0, using the packages rstatix, PMCMRplus and ggplot2.
3 Results
3.1 Physicochemical characteristics of streams
During the sampling period, the water temperature in the streams varied between 15 °C and 21 °C, with the lowest temperature observed in Haarajoki and Mustajoki, and highest in Evojoki and Majajoki (Tab. 1). On average, the water temperature in the streams was 18 °C. Streams were characterized with a slightly acidic pH, ranging from 6.1 to 6.8 (Tab. 1).
Both the water colour and DOC concentration varied significantly between streams (Tab. 2), with the lowest water colour in Ormijoki (37 mg Pt l−1) and the highest in Mustajoki (158 mg Pt l−1) (Tab. 1). DOC concentration was lowest in Luutajoki (7 mg l−1) and highest in Majajoki (17 mg l−1) (Tab. 1). The Kd at 10 cm water depth was on average 1.3 m−1, with lowest Kd observed in Luutajoki (0.2 m−1) and highest in Haarajoki (3.0 m−1) (Tab. 1). The DOC concentration explained 30% of the Kd (F1,19 = 8.183, R2 = 0.301, p = 0.01), but surprisingly, the effect of water colour on Kd was not quite significant (F1,19 = 3.353, R2 = 0.15, p = 0.083).
Water quality parameters and CHL content of Fontinalis antipyretica of study streams.
Results from the analysis of variance: interstream differences in water colour, DOC concentration and CHL a+b concentration and CHL a:b of Fontinalis antipyretica.
3.2 Chlorophyll content (CHL a+b, CHL a:b) of F. antipyretica
The CHL a+b concentration of F. antipyretica varied significantly between streams (Tab. 2), showing the highest concentration in Majajoki (1.66 μg mg−1) and the lowest in Evojoki (0.78 μg mg−1) (Tab. 1). No effect of water colour, DOC concentration or Kd on the CHL a+b concentration was observed (Tab. 3, Fig. 2).
The CHL a:b ratio of F. antipyretica also varied significantly between streams (Tab. 2), with the highest ratio observed in Luutajoki (2.26) and the lowest in Haarajoki (1.85) (Tab. 1). Neither water colour nor DOC concentration influenced the CHL a:b ratio of F. antipyretica. Instead, the ratio decreased significantly as the Kd increased (F1,19 = 10.654, R2 = 0.359, p = 0.0040) (Tab. 3, Fig. 2).
Results from the linear regression analysis: the effect of water colour, DOC concentration and Kd on CHL a+b concentration and CHL a:b of Fontinalis antipyretica.
 |
Fig. 2 Effect of water colour (left-side panels) and Kd (right-side panels) on CHL a+b concentration (upper panels) and CHL a:b (lower panels) of Fontinalis antipyretica. All data are ln-transformed. |
4 Discussion
The CHL content of F. antipyretica varied between streams, and the variation in light intensity was reflected in the CHL content, since the CHL a:b significantly decreased with increase in Kd. Whilst the total CHL a+b concentration of F. antipyretica showed significant interstream variation, this variation was not significantly affected by water colour or DOC concentration, thus contradicting with previous studies done with other macrophyte species (Barko and Filbin, 1983; Valladares and Niinemets, 2008). The increase in the CHL a+b concentration can be an adaptation mechanism of macrophytes to decreased light (Barko and Filbin, 1983; Valladares and Niinemets, 2008), but the CHL a+b concentration also expresses higher background variation − i.e. variation that is not traced to differences in water quality − due to e.g. plant age (Wood and Bachelard, 1969; Kok et al., 1990), or the changing depth distribution of photosynthetic pigments (Horppila et al., 2022). Therefore, we did not find a significant relationship between CHL a+b of F. antipyretica and the water colour, DOC or Kd.
Corroborating several previous studies (Pintado et al., 1997; Klimenko, 2012; Horppila et al., 2022; Rajala et al., 2024), the CHL a:b of F. antipyretica decreased with decreasing light availability. As sessile organisms, macrophytes have developed several mechanisms for adapting to changing light conditions, with adjusting of CHL pigments being one of the most profound (Pintado et al., 1997; Zhao et al., 2020). Under low light, an increase in the CHL b concentration enables macrophytes to be more efficient in capturing photosynthetically important short-wavelength light (Yamazaki et al., 2005). In contrast, macrophytes under high light intensity decrease the overall CHL concentration to avoid cellular damage (Kume et al., 2018).
Contrary to our expectations, water colour or the DOC concentration were not significant regulators of the CHL a:b of F. antipyretica. The CHL a:b of F. antipyretica decreased significantly only with increasing Kd between streams. DOC concentration significantly explained the variation in Kd; however, unexpectedly, no significant relationship was found between water colour and Kd in our study. This difference most likely arises from the methodological differences. Whilst water colour is determined spectrophotometrically based on the absorbance of 410 nm wavelength of light, Kd is calculated based on the entire light spectrum in the water column. Unlike DOC and Kd, water colour and Kd did not show a significant relationship, which suggests that factors influencing light attenuation outside the 410 nm wavelength affected the light attenuation in the water column. This other factor is most likely Fe which, when bound to DOC, has a strong effect on the light attenuation of short-wavelength light (Xiao et al., 2013; Horppila et al., 2023). The effect of DOC-bounded Fe on the light attenuation of short-wavelength light is not observed in water colour measurements because it is only based on the light absorption at 410 nm. Because Kd considered the whole light spectrum, the effect of DOC-bound Fe can be seen as significant. This phenomenon can be particularly prominent in high-coloured streams, where the average Fe concentrations have been shown to reach up to 1.4 mg l⁻1 (Turunen and Aroviita, 2024), being almost twice as high as in lakes (0.76 mg l⁻1) (Horppila et al., 2023). In addition, suspended solids can influence Kd especially in small streams, where the samples are taken closer to the bottom substrate than in lakes.
The differing responses in the CHL a+b/a:b content of F. antipyretica compared to previous studies done on vascular plants (Horppila et al., 2022; Rajala et al., 2024, 2025), were likely due to physiological differences in these plant groups. Bryophytes are shade-adapted plants, and the light saturation in bryophytes already occurs at moderate light levels (Glime, 2014). Compared to vascular plants, bryophytes generally have higher CHL a+b concentration and lower CHL a:b ratio (Dar et al., 2013). This trend was also observed in the current study: F. antipyretica had an average CHL a+b concentration of 0.78 μg mg−1, which is 2.69-fold higher than that of N. lutea (0.29 μg mg−1) reported by Rajala et al. (2024). Similarly, the CHL a:b ratio of F. antipyretica was approximately 25% lower compared to N. lutea (2.83) (Rajala et al., 2025). This refers to an adaptation mechanism of submerged species that enables their survival under lower light conditions (Dar et al., 2013; Estlander et al., 2025). These physiological adaptations can change the way F. antipyretica reacts to changing light conditions.
Developing a new bioindicator to monitor browning of streams is necessary because, compared to lakes, running waters are even more vulnerable, e.g. to land-use changes inducing browning (Kuglerová et al., 2021). The bioindicator should thus show a clear response specific to the environmental problem, such as increasing water colour, that it has been chosen to monitor (Cooper et al., 2009; Poikane et al., 2018). In the current study, a clear pressure-response association between CHL a:b and water colour or DOC concentration was not detected. CHL a:b of F. antipyretica indicated changes in Kd but did not reflect changes in the water colour. This hampers the feasibility of CHL a:b of F. antipyretica as a reliable indicator of browning. Instead of F. antipyretica CHL content, one suggested indicator for monitoring stream browning could be the photosynthetic pigment content of periphyton. As primary producers, the function of periphyton is controlled by light availability, and they are currently used as a biological quality element in the WFD (Sabater et al., 2007). Periphyton is considered to be a good early warning indicator of environmental changes due to acknowledged rapid response to environmental change (Montuelle et al., 2010), and therefore it would be a suitable bioindicator in streams with rapidly changing water quality.
5 Conclusions
In the current study, we did observe a significant difference in the CHL content (CHL a+b/a:b) between streams. However, neither water colour nor DOC concentration affected CHL a+b/a:b of F. antipyretica. Light attenuation coefficient (Kd) did not affect CHL a+b concentration of F. antipyretica, but the CHL a:b ratio decreased significantly with increasing Kd. DOC concentration explained one-third of the Kd, but no significant effect of water colour on Kd was found. This was most likely due to methodological differences between the water colour measurement and Kd calculation. Water colour measurements at 410 nm do not capture the effect of DOC-bound Fe, which absorbs short wavelengths but is reflected in Kd that considers the full light spectrum. As no clear pressure-response relationship between water colour or DOC concentration and CHL content of F. antipyretica was found, more research is needed to develop specific bioindicators to detect browning responses in stream environments. A potential bioindicator for stream browning could be the photosynthetic pigment content of periphyton as periphyton communities are known for rapid responses to water quality changes.
Acknowledgments
The personnel of Lammi Biological Station helped in laboratory analyses and Kari Sainio assisted with the field work.
Funding
The study was financially supported by Maa- ja Vesitekniikan tuki ry (grant number 4574) and by R. Erik and Bror Serlachius Foundation.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
The data that support the findings of this study are available from the corresponding author, S.R., upon reasonable request.
References
- Alaoui KS, Tychon B, Joachim S, Geffard A, Nott K, Ronkart S, Porcher JM, Beaudouin R, Robert C, Fauconnier ML, Saive M. 2021. Toxic effects of a mixture of five pharmaceutical drugs assessed using Fontinalis antipyretica Hedw. Ecotoxicol Environ Saf 225: 112727. [CrossRef] [PubMed] [Google Scholar]
- Albrecht E, Hannonen O, Palacin-Lizarbe C, Suni J, Härkönen L, Vainikka A, Soininen N, Kukkonen J. 2024. Browning of boreal lakes: Do public perceptions and governance meet the biological foundations? Ecol Appl 33: e2856. [Google Scholar]
- Asmala E, Carstensen J, Räike A. 2019. Multiple anthropogenic drivers behind upward trends in organic carbon concentrations in boreal rivers. Environ Res Lett 14: 124018. [CrossRef] [Google Scholar]
- Barko JW, Filbin GJ. 1983. Influences of light and temperature on chlorophyll composition in submersed freshwater macrophytes. Aquat Bot 15: 249–255. [CrossRef] [Google Scholar]
- Bhatta K, Patra HK. 2020. A review on aquatic macrophytes as bioindicators of water quality of lakes. Ecol Environ Conserv 26: 1158–1161. [Google Scholar]
- Cooper TF, Gilmour JP, Fabricius KE. 2009. Bioindicators of changes in water quality on coral reefs: review and recommendations for monitoring programmes. Coral Reefs 28: 589–606. [CrossRef] [Google Scholar]
- Dar NA, Pandit AK, Ganal BA. 2013. Seasonal variation in the pigment content of dominant macrophytes from Mular Lake, Kashmir Himalaya. India Biochem Pharmacol 2: 2167–501 [Google Scholar]
- De Wit HA, Valinia S, Weyhenmeyer GA, Futter MN, Kortelainen P, Austnes K, Hessen DO, Räike A, Laudon H, Vuorenmaa J. 2016. Current browning of surface waters will be further promoted by wetter climate. Environ Sci Technol Lett 12: 430–435. [CrossRef] [Google Scholar]
- Eloranta P. 1999. Humus and water physics. In: Keskitalo J, Eloranta P, ed. Limnology of Humic Waters. Leiden: Backhuys Publishers, 59–74. [Google Scholar]
- Estlander S, Pippingsköld E, Horppila J. 2021. Artificial ditching of catchments and brownification-connected water quality parameters of lakes. Water Res 205: 117674. [CrossRef] [PubMed] [Google Scholar]
- Estlander S, Rajala S, Pippingsköld E, Nurminen L, Horppila J. 2025. Response rate of submerged macrophyte chlorophyll content under changing light conditions. Limnology 26: 293–300. [CrossRef] [Google Scholar]
- Evans CD, Jones TG, Burden A, Ostle N, Zielinski P, Cooper MDA, Peacock M, Clark JM, Oulehle F, Cooper D, Freeman C. 2012. Acidity controls on dissolved organic carbon mobility in organic soils. Global Change Biol 18: 3317–3331. [CrossRef] [Google Scholar]
- Finnish Standards Association SFS. 1997. Water Analysis. Guidelines for the determination of total organic carbon and dissolved organic carbon. Finnish Environment Institute, Standard SFS-EN 1484. [Google Scholar]
- Finnish Standards Association SFS. 2011. Water quality. Examination and determination of colour. Finnish Environment Institute, Standard SFS-EN ISO 7887. [Google Scholar]
- Finstad AG, Andersen T, Larsen S, Tominaga K, Blumentrath S, de Wit HA, Tømmervik H, Hessen DO. 2016. From greening to browning: catchment vegetation development and reduced S-deposition promote organic carbon load on decadal time scales in Nordic lakes. Sci Rep 6: 31944. [CrossRef] [PubMed] [Google Scholar]
- Gomi T, Sidle RC, Richardson JS. 2002. Understanding processes and downstream linkages of headwater systems: headwaters differ from downstream reaches by their close coupling to hillslope processes, more temporal and spatial variation, and their need for different means of protection from land use. BioScience 52: 905–916. [CrossRef] [Google Scholar]
- Glime JM. 2014. Photosynthesis in aquatic bryophytes. In: Hanson D, Rice S, ed. Photosynthesis in Bryophytes and Early Land Plants. Advances in Photosynthesis and Respiration. Dordrecht: Springer 201–231. [CrossRef] [Google Scholar]
- Horppila J, Pippingsköld E, Estlander S. 2022. Effects of water colour on the pigment content of a floating-leaved macrophyte—implications of lake brownification. Aquat Bot 181: 10354. [Google Scholar]
- Horppila J, Keskinen S, Nurmesniemi M, Nurminen L, Pippingsköld E, Rajala S, Sainio K, Estlander S. 2023. Factors behind the threshold‐like changes in lake ecosystems along a water colour gradient: The effects of dissolved organic carbon and iron on euphotic depth, mixing depth and phytoplankton biomass. Freshwater Biol 68: 1031–1040. [CrossRef] [Google Scholar]
- Horppila J, Nurminen L, Rajala S, Estlander S. 2024. Making waves: the sensitivity of lakes to brownification and issues of concern in ecological status assessment. Water Res 249: 120964. [CrossRef] [PubMed] [Google Scholar]
- Klimenko EN. 2012. Structural and functional aspects of the Nuphar lutea (L.) Smith heterophylly: ultrastructure and photosynthesis. Cytol Genet 46: 272–279. [CrossRef] [Google Scholar]
- Kok CJ, Van der Velde G, Landsbergen KM. 1990. Production, nutrient dynamics and initial decomposition of floating leaves of Nymphaea alba L. and Nuphar lutea (L.) Smith (Nymphaeaceae) in alkaline and acid waters. Biogeochemistry 11: 235–250. [CrossRef] [Google Scholar]
- Kritzberg ES, Ekström SM. 2012. Increasing iron concentrations in surface waters − a factor behind brownification? Biogeosciences 9: 1465–1478. [CrossRef] [Google Scholar]
- Kuglerová L, Hasselquist EM, Sponseller RA, Muotka T, Hallsby G, Laudon H. 2021. Multiple stressors in small streams in the forestry context of Fennoscandia: the effects in time and space. Sci Total Environ 756: 143521. [CrossRef] [PubMed] [Google Scholar]
- Kume A, Akitsu T, Nasahara KN. 2018. Why is chlorophyll b only used in light-harvesting systems? J Plant Res 131: 961–972. [CrossRef] [PubMed] [Google Scholar]
- Li L, Zheng B, Liu L. 2010. Biomonitoring and bioindicators used for river ecosystems: Definitions, approaches and trends. Proc Environ Sci 2: 1510–1524. [CrossRef] [Google Scholar]
- Lichtenthaler HK, Wellburn AR. 1983. Determinations of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochem Soc Trans 603: 591–592. [CrossRef] [Google Scholar]
- Lyche-Solheim A, Feld CK, Birk S, Phillips G, Carvalho L, Morabito G, Mischke U, Willby N, Søndergaard M, Hellsten S, Kolada A, Mjelde M, Böhmer J, Miler O, Pusch M, Argillier C, Jeppesen E, Lauridsen TL, Poikane S. 2013. Ecological status assessment of European lakes: a comparison of metrics for phytoplankton, macrophytes, benthic invertebrates and fish. Hydrobiologia 704: 57–74. [CrossRef] [Google Scholar]
- Monteith DT, Stoddard JL, Evans CD, de Wit HA, Forsius M, Høgåsen T, Wilander A, Skjelkvåle BL, Jeffries DS, Vuorenmaa J, Keller B, Kopácek J, Vesely J. 2007. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 450: 537–540. [CrossRef] [PubMed] [Google Scholar]
- Montuelle B, Dorigo U, Bérard A, Volat B, Bouchez A, Tlili A, Gouy V, Pesce S. 2010. The periphyton as a multimetric bioindicator for assessing the impact of land use on rivers: An overview of the Ardières-Morcille experimental watershed (France). Hydrobiologia 657: 123–141. [CrossRef] [Google Scholar]
- Penning WE, Dudley B, Mjelde M, Hellsten S, Hanganu J, Kolada A, Van den Berg F M, Poikane S, Phillips G, Willby N, Ecke F. 2008. Using aquatic macrophyte community indices to define the ecological status of European lakes. Aquat Ecol 42: 253–264. [CrossRef] [Google Scholar]
- Petrin Z, Laudon H, Malmqvist B. 2007. Does freshwater macroinvertebrate diversity along a pH‐gradient reflect adaptation to low pH? Freshwater Biol 52: 2172–2183. [CrossRef] [Google Scholar]
- Pintado A, Vallandares F, Sancho LG. 1997. Exploring phenotypic plasticity in the lichen Ramalina capitata: morphology, water relations and chlorophyll content in North- and South-facing populations. Ann Bot 80: 345–353. [CrossRef] [Google Scholar]
- Poikane S, Portielje R, Denys L, Elferts D, Kelly M, Kolada A, Mäemets H, Phillips G, Søndergaard M, Willby N, Van den Berg F MS. 2018. Macrophyte assessment in European lakes: Diverse approaches but convergent views of ‘good’ ecological status. Ecol Indic 94: 185–197. [CrossRef] [PubMed] [Google Scholar]
- Proctor MC, Smirnoff N. 2011. Ecophysiology of photosynthesis in bryophytes: major roles for oxygen photoreduction and non-photochemical quenching? Physiol Plant 141: 130–140. [CrossRef] [PubMed] [Google Scholar]
- Rajala S, Estlander S, Nurminen L, Sainio K, Horppila J. 2024. Seasonal fluctuations in pigment content of macrophytes: implications for monitoring brownification. Hydrobiologia 851: 633–648. [CrossRef] [Google Scholar]
- Rajala S, Estlander S, Nurminen L, Horppila J. 2025. Spatial and temporal variation of Nuphar lutea pigment content in small boreal lakes: effect of water colour and phosphorus concentration. Hydrobiologia 852: 443–456. [CrossRef] [Google Scholar]
- Räike A, Kortelainen P, Mattsson T, Thomas DN. 2016. Long-term trends (1975-2014) in the concentrations and export of carbon from Finnish rivers to the Baltic Sea: organic and inorganic components compared. Aquat Sci 78: 505–523. [CrossRef] [Google Scholar]
- Sabater S, Guasch H, Ricart M, Romaní A, Vidal G, Klünder C, Schmitt-Jansen M. 2007. Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Anal Bioanal Chem 387: 1425–1434. [CrossRef] [PubMed] [Google Scholar]
- Scheffer M. 1998. The Ecology of Shallow Lakes. London: Chapman and Hall. [Google Scholar]
- Sepp M, Kõiv T, Nõges P, Nõges T. 2018. Do organic matter metrics included in lake surveillance monitoring in Europe provide a broad picture of brownification and enrichment with oxygen consuming substances? Sci Total Environ 610: 1288–1297. [CrossRef] [PubMed] [Google Scholar]
- Thrane JE, Hessen DO, Andersen T. 2014. The absorption of light in lakes: negative impact of dissolved organic carbon on primary productivity. Ecosystems 17: 1040–1052. [CrossRef] [Google Scholar]
- Tolkkinen MJ, Heino J, Ahonen SH, Lehosmaa K, Mykrä H. 2020. Streams and riparian forests depend on each other: A review with a special focus on microbes. For Ecol Manage 462: 117962. [CrossRef] [Google Scholar]
- Turunen J, Aroviita J. 2024. Influence of water color and catchment lake cover on stream macroinvertebrate communities: ecological insights into browning effects. Water Res 250: 121048. [CrossRef] [PubMed] [Google Scholar]
- Valladares F, Niinemets Ü. 2008. Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol Syst 39: 237–257. [CrossRef] [Google Scholar]
- Villares R, Real C, Vázquez MD. 2024. Influence of storage method on the content of photosynthetic pigments of the aquatic moss Fontinalis antipyretica. Hydrobiologia 851: 4167–4176. [CrossRef] [Google Scholar]
- Vuori KM, Muotka T. 1999. Benthic communities in humic streams. In: Keskitalo J, Eloranta P, ed. Limnology of Humic Waters. Leiden: Backhuys Publishers, 193–207 [Google Scholar]
- Weyhenmeyer GA, Karlsson J. 2009. Nonlinear response of dissolved organic carbon concentrations in boreal lakes to increasing temperatures. Limnol Oceanogr 54: 2513–2519. [CrossRef] [Google Scholar]
- Weyhenmeyer GA, Müller RA, Norman M, Tranvik LJ. 2016. Sensitivity of freshwaters to browning in response to future climate change. Clim Change 134: 225–239. [CrossRef] [Google Scholar]
- Wood JP, Bachelard EP. 1969. Variations in chlorophyll concentration in the foliage of radiata pine. Aust For 33: 119–128. [CrossRef] [Google Scholar]
- Yamazaki J, Suzuki T, Maruta E, Kamimura Y. 2005. The stoichiometry and antenna size of the two photosystems in marine green algae, Bryopsis maxima and Ulva pertusa, in relation to the light environment of their natural habitat. J Exp Bot 56: 1517–1523. [CrossRef] [PubMed] [Google Scholar]
- Zhao X, Jia T, Hu X. 2020. HCAR is a limitation factor for chlorophyll cycle and chlorophyll b degradation in chlorophyll-b-overproducing plants. Biomolecules 10. [PubMed] [Google Scholar]
- Xiao YH, Sara-Aho T, Hartikainen H, Vähätalo AV. 2013. Contribution of ferric iron to light absorption by chromophoric dissolved organic matter. Limnol Oceanogr 58: 653–662. [CrossRef] [Google Scholar]
Cite this article as: Rajala S, Estlander S, Nurminen L, Horppila J. 2025. Investigating tools for biomonitoring browning in streams: chlorophyll content of Fontinalis antipyretica. Knowl. Manag. Aquat. Ecosyst., 426. 16. https://doi.org/10.1051/kmae/2025013
All Tables
Water quality parameters and CHL content of Fontinalis antipyretica of study streams.
Results from the analysis of variance: interstream differences in water colour, DOC concentration and CHL a+b concentration and CHL a:b of Fontinalis antipyretica.
Results from the linear regression analysis: the effect of water colour, DOC concentration and Kd on CHL a+b concentration and CHL a:b of Fontinalis antipyretica.
All Figures
 |
Fig. 1 Locations of the study lakes. |
| In the text | |
 |
Fig. 2 Effect of water colour (left-side panels) and Kd (right-side panels) on CHL a+b concentration (upper panels) and CHL a:b (lower panels) of Fontinalis antipyretica. All data are ln-transformed. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.