| Issue |
Knowl. Manag. Aquat. Ecosyst.
Number 424, 2023
Freshwater ecosystems management strategies
|
|
|---|---|---|
| Article Number | 24 | |
| Number of page(s) | 10 | |
| DOI | https://doi.org/10.1051/kmae/2023021 | |
| Published online | 17 October 2023 | |
Research Paper
Effects of two subtropical piscivorous fish species on sediment disturbance and water quality: Implication for restoration by stocking piscivores
1
State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, China
2
University of Chinese Academy of Sciences, Beijing 100049, China
3
School of Civil Engineering and Architecture, Chuzhou University, Chuzhou 239000, China
4
Sino-Danish Centre for Education and Research, University of Chinese Academy of Sciences, Beijing 101408, China
5
Zhejiang Academy of Forestry, 399 Liuhe Road, 310023, Hangzhou
6
Department of Ecoscience, Aarhus University, Aarhus 8000, Denmark
7
Limnology Laboratory, Department of Biological Sciences and Centre for Ecosystem Research and Implementation, Middle East Technical University, Ankara 06800, Türkiye
8
Institute of Marine Sciences, Middle East Technical University, Mersin 33731, Türkiye
9
Institute for Ecological Research and Pollution Control of Plateau Lakes, School of Ecology and Environmental Sciences, Yunnan University, Kunming 650500, China
* Corresponding author: hehu@niglas.ac.cn; kyli@niglas.ac.cn
Received:
12
June
2023
Accepted:
21
September
2023
Subtropical lakes are often dominated by benthi-omnivorous fish (e.g., crucian carp) that may substantially disturb sediments and thereby increase water turbidity and nutrient concentrations. Piscivores stocking is sometimes used to control these benthi-omnivores in the expectation that they will reduce their adverse effects on the water quality. However, in shallow subtropical lakes the benthic foraging activities of piscivorous fish may also disturb sediments, a topic that has not yet been well elucidated. Here, we conducted a 6-day mesocosm experiment to explore the bioturbation effects of two native subtropical piscivorous fish (i.e., snakehead, Channa argus and mandarin fish, Siniperca chuatsi) in prey fish (crucian carp, Carassius carassius) present and absent scenarios. Our results showed that in mesocosms without prey, both snakehead and mandarin fish greatly increased the concentrations of suspended solids. However, the concentrations of total, organic and inorganic suspended solids were significantly higher in the mesocosms with snakehead than in those with mandarin fish, suggesting that snakehead has higher bioturbation effects than mandarin fish. When crucian carp were present, we found that both snakehead and mandarin fish significantly reduced prey abundance, whereas piscivore stocking did not significantly decrease the suspended solids and nutrient concentrations. Our study revealed differential effects of two subtropical piscivorous fish species on sediment disturbance and water quality, and we suggest that for lake restoration with piscivores stocking, mandarin fish are a better option than snakehead fish as their predation effect was equal but the strength of sediment disturbance by mandarin fish was lower than that of snakehead.
Key words: Biomanipulation / snakehead / mandarin fish / bioturbation / crucian carp
© T. Qian et al., Published by EDP Sciences 2023
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
1 Introduction
An alarming escalation of eutrophication poses a serious threat to the health and water quality of lakes around the world (Carpenter, 2005; Howarth et al., 2011; Cabrita et al., 2015). Lake eutrophication reflects excessive loading (external and internal) of nitrogen and phosphorus, which may lead to an imbalance in nutrient cycling and affect stability of the lake ecosystem (Le et al., 2010; Ferreira et al., 2011; Cheng et al., 2023). Fish are a major component of aquatic ecosystems and play a crucial role in nutrient cycling (Vanni, 2002; Weber and Brown, 2009) and ecosystem dynamics (Carpenter et al., 1985). Biomanipulation, a widely used technique in lake management, seeks to improve water transparency and ecological state (Shapiro et al., 1975; Benndorf et al., 2000), and is typically used to speed up recovery after nutrient loading reduction (Jeppesen et al., 2012). A classical biomanipulation involves stocking of piscivorous fish to control planktivorous fish with the aim to trigger a cascading top-down control on phytoplankton by predation of zooplanktivorous fish (Horppila et al., 1998; Drenner and Hambright, 1999; Carpenter et al., 2001); this technique has particularly been used in temperate lakes, although with mixed results (Lyche et al., 1990; Søndergaard et al., 1997; Skov et al., 2002).
In many subtropical shallow lakes (e.g., Wuli Lake and Huizhou West Lake in China), the fish community is dominated by benthi-omnivorous fish (e.g., crucian carp and common carp) (Xu et al., 2016; Yu et al., 2021; Guo et al., 2022). Due to the higher temperature, they have higher metabolic and excretion rates, earlier and more frequent reproduction, and smaller sizes than temperate fish species (Teixeira-de Mello et al., 2009; Jeppesen et al., 2010). One of the most detrimental effects of benthi-omnivorous fish is reworking of sediment at the sediment-water interface (bioturbation) (Meysman et al., 2006), which leads to reduced water transparency, enhanced internal nutrient loading (Breukelaar et al., 1994; Scheffer et al., 2003; Volta et al., 2013; Pledger et al., 2017), and consequently increased phytoplankton growth and eutrophication (Zambrano et al., 2001; He et al., 2017; Liu et al., 2020; Chen et al., 2021).
Whether stocking of piscivorous fish can efficiently suppress benthi-omnivorous fish in warm eutrophic lakes is not well elucidated. One uncertainty is that, even if piscivory would lead to reduction of small benthi-omnivorous fish and thereby reduce their bioturbation strength, the predation activities of piscivorous fish on benthi-omnivorous fish occur near the sediment surface in shallow lakes, which may also lead to sediment disturbance and increased turbidity (Sfakiotakis et al., 1999; He et al., 2022). For instance, Li et al. (2018) stocked mandarin fish (Siniperca chuatsi) in Biandantang Lake in Hubei Province from 2010 to 2014, and they found that the abundance and biomass of prey fish such as crucian carp (Carassius auratus) decreased significantly, but the water transparency and chlorophyll a concentration did not improve compared with the period before stocking (2006 to 2008). He et al. (2022) also found that introducing snakehead (Channa argus) significantly reduced the biomass of C. carassius, but the concentration of inorganic suspended solids increased. Catfish (Ictalurus punctatus), another potential piscivore usually preying on the sediment-water interface, has been shown to increase the concentration of TP and phytoplankton biomass in aquatic mesocosms (Parkos et al., 2003; Schrader et al., 2016). The piscivory-induced bioturbation potentially weakens the success of fish manipulation. So far, few studies have well assessed the bioturbation effects of piscivorous fish in subtropical shallow lakes (Li et al., 2018; He et al., 2022).
Here, we conducted a mesocosm experiment to compare the bioturbation effects of two ‘sit-and-wait’ piscivores (snakehead, C. argus, and mandarin fish, S. chuatsi) in the presence/absence of benthi-omnivorous fish (crucian carp, C. carassius). We hypothesized that under subtropical shallow conditions: (1) snakehead and mandarin fish would cause bioturbation in the absence of predation activity on prey fish and (2) that snakehead and mandarin fish would decrease sediment disturbance of crucian carp by preying on them, resulting in water quality improvement.
2 Materials and methods
2.1 Experimental design
The outdoor mesocosm experiment was conducted at Taihu Laboratory for Lake Ecosystem Research (31°2′1″N, 120°25′16″E) in Jiangsu Province, Eastern China from August 27 to September 1, 2022. The mesocosms consisted of 24 opaque polyethylene buckets each with 100 cm diameter at the top, 85 cm diameter at the bottom, and 83 cm depth. Before the experiment, each mesocosm received 6 cm thick sediment from a nearby pond, after which it was filled with 450 L water for a week to create a clear water state. The sediment was homogenized and sieved through a 0.5 cm sieve to remove large particles of gravel and debris. The water was directly extracted from the surface of Lake Taihu via a pump. We used juvenile crucian carp (C. carassius) as the prey of the piscivorous fish. Crucian carp is the dominant fish species in most subtropical freshwaters and poses a major threat to the water quality (Lorenz et al., 2013; Gu et al., 2018). We selected mandarin fish (S. chuatsi) and snakehead (C. argus) as piscivores as these are widely distributed in subtropical lakes of China and have been commonly used for biomanipulation (Li et al., 2018; Yu et al., 2021). All fish were obtained from a local aquaculture facility and acclimatized in cages in Lake Taihu for one month prior to the commencement of the experiment. During the acclimation period, we fed the S. chuatsi and C. argus with live crucian carp every two days.
A two-way factorial experiment was carried out with two prey levels (crucian carp absent and present, respectively) and three piscivore levels (no piscivore, mandarin fish, snakehead). Each treatment had four replicates, making up a total of 24 experimental units. After the clearwater state was established (on August 26), crucian carp were randomly stocked into half of the total mesocosms, which were set as prey present treatments (Gao et al., 2018; Gu et al., 2020). Each crucian carp-present mesocosm was stocked with 10 crucian carp corresponding to density of 10 g m−2, which is a commonly observed density in subtropical lakes (Yu et al., 2016). The remaining half of the mesocosms had no crucian carp (prey absent treatments). One day after the stocking of crucian carp, mandarin fish and snakehead (1 ind. per mesocosm) were stocked into assigned mesocosms at a density of 9 g m−2, and then the experiment began. Before introducing fish into the mesocosms, the average total length and wet weight of crucian carp, mandarin fish, and snakehead were 35.8 ± 1.5 mm and 0.56 ± 0.04 g, 78.9 ± 2.3 mm and 5.21 ± 0.17 g, and 94.5 ± 3.2 mm and 5.36 ± 0.27 g, respectively.
2.2 Sampling and monitoring
We collected 1-L samples of the water column (30 cm) daily in the morning in the middle of the mesocosm using an acrylic tube water sampler. The samples were analyzed for suspended solids. Total suspended solids (TSS) were determined by passing 500–1000 mL water samples through pre-combusted (450 °C for 2 h) and pre-weighed GF/C filters, which were then oven dried at 105 °C for 4 h and weighed followed by heating in a muffle furnace at 550 °C for 2 h to combust all organic matter and finally cooled in a desiccator to calculate the concentrations of inorganic suspended solids (ISS). Organic suspended solids (OSS) concentrations were calculated by subtracting the concentrations of ISS from TSS. On the last day of the experiment, besides suspended solids sampling, another 1-L water samples was collected for analysis of water chemicals and chlorophyll a (Chl a) concentrations. Total nitrogen (TN) and total phosphorus (TP) were examined using the unfiltered water samples by the alkaline potassium persulfate digestion-UV spectrophotometric method and the potassium persulfate oxidation-molybdenum blue colorimetric method, respectively (MEEPRC, 2002). Chl a was determined from filtered matter retained on a glass microfiber filter (Whatman GF/C International Ltd.) and extracted in a 90% acetone/water solution over 24 h, after which the concentrations were measured by a spectrophotometer (SEPA, 2002). After water sampling, we harvested all fish by emptying the water from mesocosms. The wet weight of each fish was measured. The relative growth rate (RGR) of piscivorous fish was calculated with the following equation:
RGR (% d−1) = 100 × In(Wt/W0) / days
where W0 (g) and Wt (g) are wet weight of the piscivore per mesocosm at the beginning and end of the experiment, respectively.
2.3 Statistical analyses
The R software was used for statistical analyses and graph plotting (R Core Team, 2021). We fitted generalized linear mixed models (GLMMs, Bolker et al., 2009) to explore the effects of both piscivores on suspended solids in prey present and absent scenarios. We used the interactive terms of piscivores (no piscivore, mandarin fish and snakehead) and stocking time (fixed effects) as a fixed factor in prey present and absent scenarios and included mesocosm identity as a random factor. We assumed Gaussian error distributions for all response variables and fitted modes using the glmmTMB function from the ‘glmmTMB’ package (Brooks et al., 2017). Oneway analysis of variance (one-way ANOVA) was used to analyze the TN, TP, Chl a, survival of crucian carp and RGR of piscivorous fish between treatments at the end of the experiment, with Tukey's post-hoc multiple comparisons using the TukeyHSD function from the ‘multcomp’ package (Bretz et al., 2002; Hothorn et al., 2008). All data were log-transformed to ensure normality and homoscedasticity prior to fitting to GLMMs.
3 Results
3.1 Suspended solids
At the beginning of the experiment, there were no significant differences in the concentrations of total suspended solids (TSS), inorganic suspended solids (ISS), and organic suspended solids (OSS) between the treatments (p > 0.05; Fig. 1), indicating that all experimental groups were uniform before the fish were stocked. In the prey absent mesocosms, GLMMs confirmed significant interaction effects of stocking the two piscivores and time on the TSS and ISS concentrations (p < 0.05; Tab. 1). The interaction effect of stocking snakehead and time significantly increased the concentration of OSS (p < 0.05), while no effect of stocking mandarin fish and time interaction was observed (p > 0.05; Tab. 1). At the end of the experiment, the mean concentrations of TSS, ISS, and OSS were 9.6, 19.9, and 4.4 times higher, respectively, than in those without snakehead. For mandarin fish, TSS and ISS were 3.4 and 7.3 times higher, respectively, than when absent (Fig. 1).
In the crucian carp present mesocosms, stocking of the two piscivores and time had no significant interaction effects on TSS and ISS concentrations (p > 0.05; Tab. 1). The concentrations of TSS and ISS generally increased with time in all treatments, but particularly during the first day (Fig. 1). Stocking of snakehead and time increased the OSS concentration, and their interaction was significant (p < 0.05; Tab. 1). In the mandarin fish treatment, the OSS concentration did not alter significantly with time (p > 0.05; Tab. 1).
 |
Fig. 1 Time series of total suspended solids (TSS), inorganic suspended solids (ISS), and organic suspended solids (OSS) in different treatments during the experiment. Values are means of the four replicates, and error bars represent standard error. Different letters indicate a significant difference in suspended solids concentrations at the end of the experiment, and the same letters mean not significant. Significance was set to p < 0.05. |
Summary of generalized linear mixed models results on the effect of piscivorous fish on suspended solids in prey present (PP) and absent (PA) scenarios.
3.2 Nutrients and chlorophyll a
At the end of the experiment, in the crucian carp absent scenarios, the differences between all treatments were not significant for the mean TN and Chl a concentrations (p > 0.05; Fig. 2). The mean concentration of TP in the snakehead treatment was significantly and 62.5% higher than in the treatment with mandarin fish (p < 0.05; Fig. 2). For the scenarios of crucian carp presence, there were no significant differences in the concentrations of TN, TP, and Chl a between the three treatments (p > 0.05; Fig. 2).
 |
Fig. 2 TN, TP, and Chl a concentrations in the different treatments at the end of the experiment. Values are means of the four replicates, and error bars represent standard error. Different letters indicate a significant difference in TN, TP, and Chl a concentrations, and the same letters mean not significant. Significance was set to p < 0.05. |
3.3 Fish
At the end of the experiment, densities of crucian carp were significantly lower in the mesocosms with mandarin fish and snakehead than that in those without piscivores (p < 0.001; Fig. 3). All crucian carp survived in the non-stocked piscivore treatment, while two crucian carp evaded predation in each piscivore present mesocosm (Fig. 3). The RGRs of both piscivorous fish species were significantly lower in the treatments without crucian carp than in those with prey fish (p < 0.001; Fig. 4).
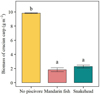 |
Fig. 3 The biomass of crucian carp in different treatments at the end of the experiment. Values are means of the four replicates, and error bars represent standard error. Different letters indicate a significant difference, and the same letters mean not significant. Significance was set to p < 0.001. |
 |
Fig. 4 RGRs of mandarin fish and snakehead in the crucian carp present and absent treatments. Values are means of the four replicates, and error bars represent standard error. Different letters indicate a significant difference, and the same letters mean not significant. Significance was set to p < 0.001. |
4 Discussion
We conducted a mesocosm experiment to compare the bioturbation effects of two native subtropical piscivores in the presence/absence of prey fish. Our results showed that when prey fish were absent, snakehead and mandarin fish disturbed the sediments and increased turbidity, but the bioturbation intensity of mandarin fish was significantly weaker than that of snakehead. When prey fish were present, both piscivores significantly reduced crucian carp density, but did not improve the water quality.
We found that when crucian carp were absent, snakehead and mandarin fish themselves caused significant sediment resuspension. Moreover, the concentration of ISS with snakehead stocking was significantly higher than in the mandarin fish treatment. This difference may be attributed to different living habits and foraging strategies of the two fish species. The mandarin fish, an ambush predator, feed exclusively on live prey (e.g., fish and shrimps) (Ding et al., 2021; Li et al., 2023) and refuse dead prey (Yi et al., 2013; Liu et al., 2015; Dou et al., 2018). When there is no live prey, the mandarin fish generally stay at the bottom and only move occasionally (Liang, 1995), implying minor bioturbation effects. The northern snakehead, another ‘sit-and-wait’ foraging fish species (Liu et al., 2000) also feed on live prey (Wang et al., 1997), but also feed on dead prey and artificial diets (Liang et al., 2001). Although we did not confirm that snakehead stirs the sediment when searching for prey, Love and Newhard (2021) observed that the northern snakehead (C. argus) preferred to burrow into the sediment, possibly as a means for escaping the heat during the summer months. On the other hand, snakehead often sticks its head out of the water surface for breathing via an accessory respiratory organ called a ‘fold gill’ (Hu and Yi, 1960) and immediately dives into the sediment if frightened (Miu, 1957). Stronger bioturbation may be caused when snakehead shuttle between the water surface and sediment compared with the less active mandarin fish. Pandian and Vivekanandan (1976) reported that starving snakehead (Ophiocephalus striatus, 750 ± 70 mg, 4.5 ± 0.5 cm) exposed to 31 cm depth of water swam more than 300 m per day during the first five days of their experiment. Thus, although both snakehead and mandarin fish may disturb the sediment, our results suggest that snakehead has a greater impact on sediment resuspension than mandarin fish when prey fish are scarce.
In the crucian carp present treatments, snakehead and mandarin fish significantly reduced crucian carp abundance. At the end of the experiment, standing crops of crucian carp in the mandarin fish and snakehead treatments were reduced by 80% to 2 g m−2. A successful biomanipulation case using piscivores stocking (i.e., pike and perch) in Lake Udbyover indicated that the final biomass of benthivorous fish (i.e., crucian carp and roach) exceeded 4 g m−2 (Skov et al., 2002), which was higher than in of our experiment (approximately 2 g m−2; Fig. 3). However, although crucian carp thus were reduced in our experiment, the water clarity and nutrient concentrations did not improve, indicating that snakehead and mandarin fish stocking were not efficient in improving water clarity on the short term, in part due to predator-induced sediment disturbance. Another mesocosm experiment with snakehead stocking (He et al., 2022) and a whole-lake biomanipulation experiment with mandarin fish (Li et al., 2018) concur with our results of poor effect on water clarity.
In both the prey-absent and the prey-present scenarios, snakehead stocking resulted in significantly higher concentrations of OSS than mandarin fish stocking, which may also be attributed to the living habits of the piscivores. Compared to the inactive mandarin fish, snakehead travelled back and forth between the sediment and water surface in our mesocosms, causing stronger sediment disturbance and thereby translocation of more nutrients from the benthic to the pelagic zones (Shormann and Cotner, 1997; Dantas et al., 2018), although TN and TP at the end of experiment were not statistically significant. The phenomenon that fish-induced sediment resuspension increases algal concentrations has been well elucidated in many benthi-omnivorous fish (e.g., common carp and crucian carp) studies (Roozen et al., 2007; Badiou and Goldsborough, 2015; He et al., 2017; Han et al., 2022). Our study showed that the non-algal turbidity was augmented by benthi-piscivorous fish in the absence of predation. It is worth noting that regardless of whether prey fish were present or not, snakehead increased algal turbidity.
Our study showed clear bioturbation effects of subtropical piscivorous fishes. Therefore, cautions should be taken in lake restoration when using piscivore stocking. Our results also showed that the strength of sediment disturbance caused by mandarin fish was lower than that of snakehead fish. However, being a mesocosm study, our experiment has clear weaknesses concerning both scale and duration. The small size of the mesocosm (about 0.5 m3) may have exaggerated the extent of sediment disturbance and the short duration of the experiment may have increased the predation loss of prey. Follow-up large-scale experiments should be conducted to fully elucidate the role of the subtropical freshwater piscivores.
Funding
This study was financially supported by National Science Foundation of China (No. 31971473; 31930074), and the “Pioneer” and “Leading Goose” R&D Program of Zhejiang Province, China (2022C02038) and Jiangxi Provincial Natural Science Foundation (20232ACB215006). EJ was supported by AQUACOSM (Network of Leading European AQUAtic MesoCOSM Facilities Connecting Mountains to Oceans from the Arctic to the Mediterranean), AnaEE Denmark (anaee.dk), and the Tübitak program BIDEB 2232 (project 118C250).
Declaration of competing interest
None declared.
Data availability statement
All data were present in the paper.
Acknowledgements
All experimental procedures involving fish were approved by the Institutional Animal Care and Use Committee of the Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences. We would like to thank the Taihu Laboratory for Lake Ecosystem Research for providing us with the experimental facility for this study. We also express our gratitude to Anne Mette Poulsen for linguistic assistance.
References
- Badiou PHJ, Goldsborough LG. 2015. Ecological impacts of an exotic benthivorous fish the common carp (Cyprinus carpio L.), on water quality, sedimentation, and submerged macrophyte biomass in wetland mesocosms. Hydrobiologia 755: 107–121. [CrossRef] [Google Scholar]
- Benndorf J, Wissel B, Sell AF, Hornig U, Ritter P, Boïng W. 2000. Food web manipulation by extreme enhancement of piscivory: an invertebrate predator compensates for the effects of planktivorous fish on a plankton community. Limnologica 30: 235–245. [CrossRef] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24: 127–135. [CrossRef] [PubMed] [Google Scholar]
- Bretz F, Hothorn T, Westfall P. 2002. On multiple comparisons in R. R-news 3: 314–317. [Google Scholar]
- Breukelaar AW, Lammens EHRR, Breteler JPG, Tátrai I. 1994. Effects of benthivorous bream (Abramis brama) and carp (Cyprinus carpio) on sediment resuspension and concentration of nutrients and chlorophyll a. Freshw Biol 32: 113–121. [CrossRef] [Google Scholar]
- Brooks ME, Kristensen K, Van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9: 378–400. [CrossRef] [Google Scholar]
- Cabrita MT, Silva A, Oliveira PB, Angélico MM, Nogueira M. 2015. Assessing eutrophication in the Portuguese continental Exclusive Economic Zone within the European Marine Strategy Framework Directive. Ecol Indic 58: 286–299. [CrossRef] [Google Scholar]
- Carpenter SR, Kitchell JF, Hodgson JR. 1985. Cascading trophic interactions and lake productivity. BioScience 35: 634–639. [CrossRef] [Google Scholar]
- Carpenter SR, Cole JJ, Hodgson JR, Kitchell JF, Pace ML, Bade D, Cottingham KL, Essington TE, Houser JN, Schindler DE. 2001. Trophic cascades, nutrients, and lake productivity: whole-lake experiments. Ecol Monogr 71: 163–186. [CrossRef] [Google Scholar]
- Carpenter SR. 2005. Eutrophication of aquatic ecosystems: bistability and soil phosphorus. Proc Natl Acad Sci 102: 10002–10005. [CrossRef] [PubMed] [Google Scholar]
- Chen KQ, Li QS, Han YQ, Li W, Zhang Y, Li KY, He H. 2021. Effects of Carassius auratus on water turbidity, nutrient levels and plankton biomass in shallow lakes: a mesocosm experiment. J Lake Sci 33: 397–404 (in Chinese with English abstract). [CrossRef] [Google Scholar]
- Cheng LJ, Gao XY, Wang GW, Ding Z, Xue B, Zhang C, Liu JL, Jiang QF. 2023. Intensified sensitivity and adaptability of zooplankton Bosminidae in subtropical shallow freshwater lakes with increasing trophic level. Front Ecol Evol 11: 1121632. [CrossRef] [Google Scholar]
- Dantas DDF, Rubim PL, de Oliveira FA, da Costa MRA, de Moura CGB, Teixeira LH, Attayde JL. 2018. Effects of benthivorous and planktivorous fish on phosphorus cycling, phytoplankton biomass and water transparency of a tropical shallow lake. Hydrobiologia 829: 31–41. [Google Scholar]
- Ding WD, Zhang XH, Zhao XM, Jing W, Cao ZM, Li J, Huang Y, You XX, Wang M, Shi Q. 2021. A chromosome-level genome assembly of the mandarin fish (Siniperca chuatsi). Front Genet 12: 671650. [CrossRef] [PubMed] [Google Scholar]
- Dou YQ, He S, Liang XF, Cai WJ, Wang J, Shi LJ, Li J. 2018. Memory function in feeding habit transformation of mandarin fish (Siniperca chuatsi). Int J Mol Sci 19: 1254. [CrossRef] [PubMed] [Google Scholar]
- Drenner RW, Hambright KD. 1999. Biomanipulation of fish assemblages as a lake restoration technique. Arch Hydrobiol 146: 129–165. [CrossRef] [Google Scholar]
- Ferreira JG, Andersen JH, Borja A, Bricker SB, Camp J, Cardoso da Silva M, Garcés E, Heiskanen AS, Humborg C, Ignatiades L, Lancelot C, Menesguen A, Tett P, Hoepffner N, Claussen U. 2011. Overview of eutrophication indicators to assess environmental status within the european marine strategy framework directive. Estuar Coast Shelf Sci 93: 117–131. [CrossRef] [Google Scholar]
- Gao J, Zhong P, Ning JJ, Liu ZW, Jeppesen E. 2018. Herbivory of omnivorous fish shapes the food web structure of a Chinese tropical eutrophic lake: evidence from stable isotope and fish gut content analyses. Water 9: 69. [Google Scholar]
- Gu J, He H, Jin H, Yu JL, Jeppesen E, Nairn RW, Li KY. 2018. Synergistic negative effects of small-sized benthivorous fish and nitrogen loading on the growth of submerged macrophytes-relevance for shallow lake restoration. Sci Total Environ 610: 1572–1580. [CrossRef] [PubMed] [Google Scholar]
- Gu J, Li KY, Jeppesen E, Han YQ, Jin H, He H, Ning XY. 2020. Using freshwater bivalves (Corbicula Fluminea) to alleviate harmful effects of small-sized crucian carp (Carassius Carassius) on growth of submerged macrophytes during lake restoration by biomanipulation. Water 12: 3161. [CrossRef] [Google Scholar]
- Guo C, Li W, Li SQ, Mai Z, Zhang TL, Liu JS, Hansen AG, Li L, Cai XW, Hicks BJ. 2022. Manipulation of fish community structure effectively restores submerged aquatic vegetation in a shallow subtropical lake. Environ Pollut 292: 118459. [CrossRef] [PubMed] [Google Scholar]
- Han YQ, Zhang, Y, Li QS, Lurling M, Li W, He H, Gu J, Li KY. 2022. Submerged macrophytes benefit from lanthanum modified bentonite treatment under juvenile omni-benthivorous fish disturbance: implications for shallow lake restoration. Freshw Biol 67: 672–683. [CrossRef] [Google Scholar]
- He H, Hu E, Yu JL, Luo XG, Li KY, Jeppesen E, Liu ZW. 2017. Does turbidity induced by Carassius carassius limit phytoplankton growth? A mesocosm study. Environ Sci Pollut R 24: 5012–5018. [CrossRef] [PubMed] [Google Scholar]
- He H, Qian T, Shen RJ, Yu JL, Li KY, Liu ZW, Jeppesen E. 2022. Piscivore stocking significantly suppresses small fish but does not facilitate a clear-water state in subtropical shallow mesocosms: a biomanipulation experiment. Sci Total Environ 842: 156967. [CrossRef] [PubMed] [Google Scholar]
- Horppila J, Peltonen H, Malinen T, Luokkanen E, Kairesalo T. 1998. Top-down or bottom-up effects by fish: issues of concern in biomanipulation of lakes. Restor Ecol 6: 20–28. [CrossRef] [Google Scholar]
- Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom J 50: 346–363. [CrossRef] [MathSciNet] [PubMed] [Google Scholar]
- Howarth R, Chan F, Conley DJ, Garnier J, Doney SC, Marino R, Billen G. 2011. Coupled biogeochemical cycles: eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front Ecol Environ 9: 18–26. [CrossRef] [Google Scholar]
- Hu CK, Yi MC. 1960. The habits, catching and breeding of snakehead. Chinese J Zool 7: 321–323 (in Chinese). [Google Scholar]
- Jeppesen E, Meerhoff M, Holmgren K, Gonzalez-Bergonzoni I, Teixeira-de Mello F, Declerck SAJ, De Meester L,Søndergaard M, Lauridsen TL, Bjerring R, Conde-Porcuna JM, Mazzeo N,Iglesias C, Reizenstein M, Malmquist HJ, Liu ZW, Balayla D, Lazzaro X. 2010. Impacts of climate warming on lake fish community structure and potential ecosystem effects. Hydrobiologia 646: 73–90. [CrossRef] [Google Scholar]
- Jeppesen E, Mehner T, Winfield IJ, Kangur K, Sarvala J, Gerdeaux D, Rask M, Malmquist HJ, Holmgren K, Volta P, Romo S, Eckmann R, Sandstrom A, Blanco S, Kangur A, Stabo HR, Tarvainen M, Ventela AM, Søndergaard M, Lauridsen TL, Meerhoff M. 2012. Impacts of climate warming on the long-term dynamics of key fish species in 24 European lakes. Hydrobiologia 694: 1–39. [CrossRef] [Google Scholar]
- Le C, Zha Y, Li Y, Sun D, Lu H, Yin B. 2010. Eutrophication of Lake Waters in China: Cost, Causes, and Control. Environ Manage 45: 662–668. [CrossRef] [PubMed] [Google Scholar]
- Li L, Tang SL, He S, Liang XF. 2023. Transcriptome analysis provides an overview of genes involved in the peculiar food preference at first-feeding stage in mandarin fish (Siniperca chuatsi). Fishes 8: 17. [Google Scholar]
- Li W, Hicks BJ, Lin M, Guo C, Zhang T, Liu J, Beauchamp DA. 2018. Impacts of hatchery-reared mandarin fish Siniperca chuatsi stocking on wild fish community and water quality in a shallow Yangtze lake. Sci Rep 8: 11481. [CrossRef] [PubMed] [Google Scholar]
- Liang XF. 1995. Visual characteristics of mandarin fish (Siniperca chuatsi) in relation to its feeding habit Ⅲ. Visual response to prey motion and shape. Acta Hydrobiologica Sinica 19: 70–75 ( in Chinese with English abstract). [Google Scholar]
- Liang XF, Oku H, Ogata HY, Liu J, He X. 2001. Weaning Chinese perch Siniperca chuatsi (Basilewsky) onto artificial diets based upon its specific sensory modality in feeding. Aquac Res 32: 76–82. [CrossRef] [Google Scholar]
- Liu JS, Cui YB, Liu JK. 2000. Resting metabolism and heat increment of feeding in mandarin fish (Siniperca chuatsi) and Chinese snakehead (Channa argus). Comp Biochem Physiol A Mol Integr Physiol 127: 131–138. [CrossRef] [PubMed] [Google Scholar]
- Liu LW, Liang XF, Fang JG. 2015. The optimal stocking density for hybrid of Siniperca chuatsi (♀) × Siniperca scherzeri (♂) mandarin fish fed minced prey fish. Aquac Res 48: 1342–1345. [Google Scholar]
- Liu ZW, Zhang XF, Chen FZ, Du YX, Guan BH, Yu JL, He H, Zhang YD. 2020. The responses of the benthic-pelagic coupling to eutrophication and regime shifts in shallow lakes: implication for lake restoration. J Lake Sci 32: 1–10 (in Chinese with English abstract). [CrossRef] [Google Scholar]
- Lorenz AW, Stoll S, Sundermann A, Haase P. 2013. Do adult and YOY fish benefit from river restoration measures? Ecol Eng 61: 174–181. [CrossRef] [Google Scholar]
- Love JW, Newhard JJ. 2021. Using published information to predict consumption by northern snakehead in Maryland. T Am Fish Soc 150: 425–434. [CrossRef] [Google Scholar]
- Lyche A, Faafeng BA, Brabrand A. 1990. Predictability and possible mechanisms of plankton response to reduction of piscivorous fish. Hydrobiologia 200: 251–261. [CrossRef] [Google Scholar]
- MEEPRC (Ministry of Ecology and Environment, PRC). 2002. Methods for the examination of water and wastewater. Beijing: China Environmental Science Press, 836 p. ( in Chinese). [Google Scholar]
- Meysman FJR, Galaktionov OS, Gribsholt B, Middelburg J, 2006. Bioirrigation in permeable sediments: advective pore-water transport induced by burrow ventilation. Limnol Oceanogr 51: 142–156. [CrossRef] [Google Scholar]
- Miu LX. 1957. The habits and catching methods of snakehead. Biology of Bulletin 11: 35–37 (in Chinese). [Google Scholar]
- Pandian TJ, Vivekanandan E. 1976. Effects of feeding and starvation on growth and swimming activity in an obligatory air-breathing fish. Hydrobiologia 49: 33–39. [CrossRef] [Google Scholar]
- Parkos JJ, Santucci VJ, Wahl DH. 2003. Effects of adult common carp (Cyprinus carpio) on multiple trophic levels in shallow mesocosms. Can J Fish Aquat Sci 60: 182–192. [Google Scholar]
- Pledger A, Rice S, Millett J. 2017. Foraging fish as zoogeomorphic agents: an assessment of fish impacts at patch, barform, and reach scales. J Geophys Res-earth 122: 2105–2123. [CrossRef] [Google Scholar]
- R Core Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Roozen FCJM, Lurling M, Vlek H, Kraan EAJVP, Ibelings BW, Scheffer M. 2007. Resuspension of algal cells by benthivorous fish boosts phytoplankton biomass and alters community structure in shallow lakes. Freshw Biol 52: 977–987. [CrossRef] [Google Scholar]
- Scheffer M, Portielje R, Zambrano L. 2003. Fish facilitate wave resuspension of sediment. Limnol Oceanogr 48: 1920–1926. [CrossRef] [Google Scholar]
- Schrader KK, Tucker CS, Brown TW, Torrans EL, Whitis GN. 2016. Comparison of phytoplankton communities in catfish split-pond aquaculture systems with conventional ponds. N Am J Aquacult 78: 384–395. [CrossRef] [Google Scholar]
- SEPA. 2002. Analytical methods for water and wastewater monitor, 4th ed. Beijing: Chinese Environmental Science Press, ( in Chinese). [Google Scholar]
- Sfakiotakis M, Lane DM, Davies JBC. 1999. Review of fish swimming modes for aquatic locomotion. IEEE J Oceanic Eng 24: 237–252. [CrossRef] [Google Scholar]
- Shapiro J, Lamarra V, Lynch M. 1975. Biomanipulation: an ecosystem approach to lake restoration. In Brezonik PL, Fox JL, eds. Proceedings of a Symposium on Water Quality Management Through Biological Control, Gainesville, Florida: University of Florida Gainesville pp. 85–96. [Google Scholar]
- Shormann DE, Cotner JB. 1997. The effects of benthivorous smallmouth buffalo (Ictiobus bubalus) on water quality and nutrient cycling in a shallow floodplain lake. Lake Reserv Manage 13: 270–278. [CrossRef] [Google Scholar]
- Skov C, Perrow MR, Berg S, Skovgaard H. 2002. Changes in the fish community and water quality during seven years of stocking piscivorous fish in a shallow lake. Freshw Biol 47: 2388–2400. [CrossRef] [Google Scholar]
- Søndergaard M, Jeppesen E, Berg S. 1997. Pike (Esox lucius L.) stocking as a biomanipulation tool. 2. Effects on lower trophic levels in Lake Lyng, Denmark. Hydrobiologia 342: 319–325. [CrossRef] [Google Scholar]
- Teixeira-de Mello F, Meerhoff M, Pekcan-Hekim Z, Jeppesen E. 2009. Substantial differences in littoral fish community structure and dynamics in subtropical and temperate shallow lakes. Freshw Biol 54: 1202–1215. [CrossRef] [Google Scholar]
- Vanni MJ. 2002. Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Syst 33: 341–370. [CrossRef] [Google Scholar]
- Volta P, Jeppesen E, Leoni B, Campi B, Sala P, Garibaldi L, Lauridsen T, Winfield IJ. 2013. Recent invasion by a non-native cyprinid (common bream Abramis brama) is followed by major changes in the ecological quality of a shallow lake in southern Europe. Biol Invasions 15: 2065–2079. [CrossRef] [Google Scholar]
- Weber MJ, Brown ML. 2009. Effects of common carp on aquatic ecosystems 80 years after Carp as a dominant: ecological insights for fisheries management. Rev Fish Sci Aquac 17: 524–537. [CrossRef] [Google Scholar]
- Xu J, Zhang H, Cai Y, García Molinos J, Zhang M. 2016. Optimal response to habitat linkage of local fish diversity and mean trophic level. Limnol Oceanogr 61: 1438–1448. [CrossRef] [Google Scholar]
- Yi TL, Sun J, Liang XF, He S, Li L, Wen ZY, Shen D. 2013. Effects of polymorphisms in pepsinogen (PEP), amylase (AMY) and trypsin (TRY) genes on food habit domestication traits in mandarin fish. Int J Mol Sci 14: 21504–21512. [CrossRef] [PubMed] [Google Scholar]
- Yu JL, Liu ZW, He H, Zhen W, Guan BH, Chen FZ, Li KY, Zhong P, de Mello FT, Jeppesen E. 2016. Submerged macrophytes facilitate dominance of omnivorous fish in a subtropical shallow lake: implications for lake restoration. Hydrobiologia 775: 97–107. [CrossRef] [Google Scholar]
- Yu JL, Zhen W, Kong L, He H, Zhang YD, Yang XD, Chen FZ, Zhang M, Liu ZW, Jeppesen E. 2021. Changes in pelagic fish community composition, abundance, and biomass along a productivity gradient in subtropical lakes. Water 13: 858. [CrossRef] [Google Scholar]
- Zambrano L, Scheffer M, Martinez-Ramos M. 2001. Catastrophic response of lakes to benthivorous fish introduction. Oikos 94: 344–350. [CrossRef] [Google Scholar]
Cite this article as: Qian T, He H, Li K, Yue C, Jeppesen E. 2023. Effects of two subtropical piscivorous fish species on sediment disturbance and water quality: Implication for restoration by stocking piscivores. Knowl. Manag. Aquat. Ecosyst., 424, 24.
All Tables
Summary of generalized linear mixed models results on the effect of piscivorous fish on suspended solids in prey present (PP) and absent (PA) scenarios.
All Figures
 |
Fig. 1 Time series of total suspended solids (TSS), inorganic suspended solids (ISS), and organic suspended solids (OSS) in different treatments during the experiment. Values are means of the four replicates, and error bars represent standard error. Different letters indicate a significant difference in suspended solids concentrations at the end of the experiment, and the same letters mean not significant. Significance was set to p < 0.05. |
| In the text | |
 |
Fig. 2 TN, TP, and Chl a concentrations in the different treatments at the end of the experiment. Values are means of the four replicates, and error bars represent standard error. Different letters indicate a significant difference in TN, TP, and Chl a concentrations, and the same letters mean not significant. Significance was set to p < 0.05. |
| In the text | |
 |
Fig. 3 The biomass of crucian carp in different treatments at the end of the experiment. Values are means of the four replicates, and error bars represent standard error. Different letters indicate a significant difference, and the same letters mean not significant. Significance was set to p < 0.001. |
| In the text | |
 |
Fig. 4 RGRs of mandarin fish and snakehead in the crucian carp present and absent treatments. Values are means of the four replicates, and error bars represent standard error. Different letters indicate a significant difference, and the same letters mean not significant. Significance was set to p < 0.001. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


