| Issue |
Knowl. Manag. Aquat. Ecosyst.
Number 417, 2016
|
|
|---|---|---|
| Article Number | 24 | |
| Number of page(s) | 4 | |
| DOI | https://doi.org/10.1051/kmae/2016011 | |
| Published online | 19 April 2016 | |
Short communication
Dominance of Myriophyllum spicatum in submerged macrophyte communities associated with grass carp
La dominance de Myriophyllum spicatum dans les communautés de macrophytes immergées associées à la carpe amour
1
State Key Laboratory of Lake Science and Environment, Nanjing
Institute of Geography and Limnology, Chinese Academy of Sciences,
Nanjing
210008, P.R.
China
2
Department of Ecology and Institute of Hydrobiology, Jinan
University, Guangzhou
510630, P.R.
China
3
Sino-Danish Centre for Education and Research (SDC),
Beijing, P.R.
China
4
Department of Bioscience, Aarhus University,
8600
Silkeborg,
Denmark
⋆ Corresponding author:
jlyu@niglas.ac.cn
Received: 5 January 2016
Revised: 18 March 2016
Accepted: 20 March 2016
Re-establishment of macrophyte communities is a key to restore eutrophic shallow lakes. The species composition of the plant communities may change rapidly during the recovery period. A basin in subtropical Lake Qinhu (China) was restored by biomanipulation including fish removal followed by planting of submerged macrophytes in 2011. In September-December 2011, dominance of Vallisneria spinulosa and Ceratophyllum demersum shifted to dominance of Myriophyllum spicatum. Meanwhile, the CPUE (catch per unit effort) showed that the number and biomass of grass carp (Ctenopharyngodon idella) demonstrated a marked increase. Stable isotope analyses revealed that grass carp preferred V. spinulosa and C. demersum to M. spicatum. We propose that grazing by grass carp was responsible for the observed shift in dominance towards M. spicatum but as alternative explanations are possible, further tests by controlled experiments are needed to draw firm conclusions.
Résumé
La re-création de communautés de macrophytes est une clé pour restaurer les lacs peu profonds eutrophes. La composition des espèces des communautés végétales peut changer rapidement au cours de la période de récupération. Un bassin subtropical du lac Qinhu (Chine) a été restauré par biomanipulation avec l’enlèvement de poisson suivi par la plantation de macrophytes submergés en 2011. En septembre-décembre 2011, la prédominance des Vallisneria spinulosa et Ceratophyllum demersum s’est déplacée vers une dominance de Myriophyllum spicatum. Pendant ce temps, les CPUE (captures par unité d’effort) ont montré que le nombre et la biomasse de la carpe herbivore (Ctenopharyngodon idella) ont présenté une augmentation marquée. Les analyses des isotopes stables ont révélé que la carpe préfère V. spinulosa et C. demersum à M. spicatum. Nous proposons que le pâturage par la carpe amour fût responsable du changement observé dans la dominance vers M. spicatum mais comme d’autres explications sont possibles, d’autres tests par des expériences contrôlées sont nécessaires pour tirer des conclusions définitives.
Key words: grass carp / food preference / submerged macrophytes / community structure / stable isotope
Mots clés : carpe herbivore / préférence alimentaire / macrophyte submergé / structure de communauté / isotopes stables
© J. Yu et al., published by EDP Sciences, 2016
 This is an Open Access article distributed under the terms of the Creative
Commons Attribution License CC-BY-ND (http://creativecommons.org/licenses/by-nd/4.0/), which permits
unrestricted use, distribution, and reproduction in any medium, provided the original
work is properly cited. If you remix, transform, or build upon the material, you may not
distribute the modified material.
This is an Open Access article distributed under the terms of the Creative
Commons Attribution License CC-BY-ND (http://creativecommons.org/licenses/by-nd/4.0/), which permits
unrestricted use, distribution, and reproduction in any medium, provided the original
work is properly cited. If you remix, transform, or build upon the material, you may not
distribute the modified material.
Submerged macrophytes play an important role in structuring aquatic ecosystems (Jeppesen et al., 1998) and help maintaining clear water conditions (Scheffer and Jeppesen, 1998) by reducing resuspension (James et al., 2004), suppressing algal growth (Gross, 2003), and providing a refuge for zooplankton against predation by planktivorous fish (Lauridsen and Lodge, 1996). Therefore, re-establishment of macrophyte communities is a key to restore eutrophic shallow lakes (Jeppesen et al., 2012). Different species of macrophytes likely have different effects on lake ecosystems. Vallisneria has a higher ratio of roots to total biomass and thus stronger effects on sediment-water interactions than less rooted species such as Elodea (Zhang et al., 2010); therefore, particularly Vallisneria is often planted when restoring eutrophic shallow lakes in China (Chen et al., 2010; Zhang et al., 2012; Liu et al., 2014). A basin (mean depth 1.5 m, surface area 8 ha) in subtropical Lake Qinhu was restored by biomanipulation including fish removal followed by planting of submerged macrophytes (Vallisneria spinulosa, Hydrilla verticillata, Myriophyllum spicatum, and Ceratophyllum demersum) in Spring of 2011. After this, submerged macrophyte flourished and a clear water state was established in the restored basin. Here we reported a shift in dominance of V. spinulosa and C. demersum to dominance of M. spicatum occurring in September-December 2011 associated with a major increase in abundance of grass carp. We also studied the feeding habits of grass carp in December using stable isotope analyses and community changes of fish associated with the changes in macrophytes in order to explore the possible interactions between macrophytes and these fish.
The physical and chemical parameters of the lake were measured at three different sites along a mid-lake gradient. Water temperature (WT), dissolved oxygen (DO) and pH were measured using a YSI sonde (YSI 6500, YSI Company, USA). A Secchi disk was used to measure standard depth (SD), and water depth (WD) was recorded with a sensor (SM-5, Speedtech Instruments, USA). Total nitrogen (TN), total phosphorus (TP), total suspended solids (TSS) and chlorophyll a (Chl a) were determined using Chinese standard methods (Jin and Tu, 1990). The submerged macrophyte community was investigated with a 0.25 m2 clamp at four lake sites located along a mid-lake gradient in the restored part, and two samples were taken at each side of a boat at each sampling site. Fish were collected using a 80 ×1.5 m gillnet with multiple mesh sizes: 10, 15, 25 and 40 mm at each sampling event, one gillnet being set at the central area of the lake in the morning (around 9:00 a.m) and retrieved after 2 h.
For stable isotope analyses, the leaves of the macrophytes (one sample per site of each species) were washed repeatedly with distilled water in the laboratory to ensure that all the attached organic matter was removed, after which the plants were oven-dried at 60 °C for 36–48 h for further stable carbon and nitrogen analysis. For fish samples, the dorsal white muscle was removed and dried at 60 °C for further stable isotope analysis.
Fish and macrophyte samples were analyzed to determine 13C/12C and 15N/14N ratios using a SerCon 20-20
isotope ratio mass spectrometer at the Department of Ecology and Institute of Hydrobiology,
Jinan University, Guangzhou, China. Isotope abundance was expressed using the conventional
delta notation against conventional international standards (Pee Dee Belemnite for
δ13C
and atmospheric nitrogen for δ15N): 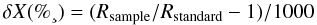 where
where 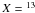 C or 15N and R is the ratio of 13C:12C or 15N:14N. The precision of repeated
measurements was ca.±0.3%.
C or 15N and R is the ratio of 13C:12C or 15N:14N. The precision of repeated
measurements was ca.±0.3%.
To determine the relative contribution of different primary producers to the diet of consumers, an isotopic mixing model, IsoSource (Phillips and Gregg, 2003), was used. In our study, the four sampling sites were regarded as replicates, and the variations in the submerged macrophyte community between the two months were analyzed by one-way ANOVA. All comparisons were made with the statistical package SPSS version 22.0 (IBM Corporation, Somers, New York, USA).
The physical and chemical data on the restored basin of Lake Qinhu for the two sampling periods are given in Table 1. The water of the basin was clear with generally low concentrations of TSS, Chl a, TN, and TP. The water temperature in December was significantly lower than that in September (P< 0.001), while the DO concentration was higher in September (P< 0.05); no significant differences were found for the other physic-chemical parameters (Table 1).
Physical and chemical parameters of the restored part of Lake Qinhu during the sampling months.
The composition and total biomass of submerged macrophytes changed significantly from September to December. In September, C. demersum and V. spinulosa were the dominant submerged macrophyte species, while M. spicatum constituted, on average, <2% of the biomass (Figure 1) (managers harvested H. verticillata). However, in December, M. spicatum became the dominant submerged macrophyte (>95% of biomass) (Figure 1), while the average biomass of C. demersum and V. spinulosa constituted <10%. The total biomass of submerged macrophytes also declined significantly (P< 0.05) (Figure 1).
 |
Fig. 1 Composition and total biomass of submerged macrophytes in the restored part of Lake Qinhu in September and December. Error bars represent the standard deviation (SD) of different sampling sites. |
 |
Fig. 2 Structure of the fish community in the restored part of Lake Qinhu in September and December, 2011. (A) total fish number per unit effort, NPUE; (B) total fish biomass per unit effort, BPUE; (C) per cent abundance of each fish species; (D) per cent biomass of each fish species. |
 |
Fig. 3 Stable isotopic signatures of grass carp and macrophytes (A) and the per cent contribution of macrophytes to the carbon source of grass carp (B) estimated using the IsoSource model. Error bars represent the standard deviation (SD) of different individuals. |
A total of six fish species were caught in the restored part of Lake Qinhu, namely silver carp Hypophthalmichthys molitrix, bighead carp Hypophthalmichthys nobilis, sharpbelly Hemicculter leuciclus, crucian carp Carassius auratus, common carp Cyprinus carpio, and grass carp Ctenopharyngodon idella. The total number (NPUE, Figure 2A) and biomass (BPUE, Figure 2B) of fish caught increased from September to December. In September, the omni-planktivorous species, sharpbelly and bighead carp, dominated the fish community in both abundance and biomass (Figure 2, C and D), whereas no grass carp were caught. However, in December, grass carp constituted 44% of total abundance and 46% of total biomass, respectively (Figures 2C and 2D).
The carbon stable isotope ratio (δ13C) was closer to the values of V. spinulosa and C. demersum than to M. spicatum (Figure 3A). IsoSource model estimation revealed that the relative contributions of V. spinulosa and C. demersum to the diet of grass carp were 54.8% and 40.3%, respectively, while the contribution of M. spicatum was only 4.9% (Figure 3B), suggesting that the grazing intensity of grass carp on V. spinulosa and C. demersum was much higher than that on M. spicatum.
Pípalová (2002) showed a shift from dominance of Eleocharis acicularis, Potamogeton pusillus and P. pectinatus to M. spicatum after grass carp stocking. High phenolic concentration of M. spicatum may discourage grass carp feeding (Dorenbosch and Bakker, 2011), resulting in a lower grazing effect on this plant species. We therefore propose that grazing by grass carp was responsible for the observed shift in dominance towards M. spicatum but the changes could potential also reflect natural seasonal variations. However studies in natural (Liu et al., 2007) and biomanipulated subtropical shallow lake (Guan et al., 2011, unpublished data), located in the same climatic zone as Lake Qinhu, showed consistent composition and high biomass of submerged macrophytes in September-December. Nevertheless, further tests by controlled experiments are needed to draw firm conclusions.
Acknowledgments
We thank Xu Wang and Ming Zhang for field and laboratory support and Anne Mette Poulsen for language assistance. Thanks also to the anonymous reviewers for their very constructive and helpful comments. This study was supported by the National Natural Science Foundation of China (31400400, 31270409) and the Key Project of 135 Program of Nanjing Institute of Geography and Limnology (NIGLAS2012135007, NIGLAS2012135002). E.J. was supported by the MARS project (Managing Aquatic ecosystems and water Resources under multiple Stress) funded under the 7th EU Framework Programme, Theme 6 (Environment including Climate Change), Contract No.: 603378 (http://www.mars-project.eu), ‘CLEAR’ (a Villum Kann Rasmussen Centre of Excellence project), and CRES.
References
- Chen L., Zhang X. and Liu Z., 2010. The response of a phytoplankton community to ecosystem restoration in Huizhou West Lake. J. Wuhan Bot. Res., 28, 453–459. (In Chinese with English abstract, DOI: 10.3724/SP.J.1142.2010.40453). [Google Scholar]
- Dorenbosch M. and Bakker E.S., 2011. Herbivory in omnivorous fishes: effect of plant secondary metabolites and prey stoichiometry. Freshwater Biol., 56, 1783–1797. [CrossRef] [Google Scholar]
- Gross E.M., 2003. Allelopathy of aquatic autotrophs. Crit. Rev. Plant Sci., 22, 313–339. [Google Scholar]
- James W.F., Barko J.W. and Butler M.G., 2004. Shear stress and sediment resuspension in relation to submerged macrophyte biomass. Hydrobiologia, 515, 181–191. [CrossRef] [Google Scholar]
- Jeppesen E., Lauridsen T.L., Kairesalo T. and Perrow M.R., 1998. Impact of submerged macrophytes on fish-zooplankton interactions in lakes. In: Jeppesen, E., Søndergaard Ma., Søndergaard M. and Christoffersen K. (eds.), The Structuring Role of Submerged Macrophytes in Lakes, Springer Verlag, New York, 91–114. [Google Scholar]
- Jeppesen E., Søndergaard M., Lauridsen T.L., Davidson T.A., Liu Z.W., Mazzeo N., Trochine C., Özkan K., Jensen H.S., Trolle D., Starling F., Lazzaro X., Johansson L.S., Bjerring R., Liboriussen L., Larsen S.E., Landkildehus F. and Meerhoff M., 2012. Biomanipulation as a restoration tool to combat eutrophication: recent advances and future challenges. Advan. Ecol. Res., 47, 411–487. [CrossRef] [Google Scholar]
- Jin X.C. and Tu Q.Y., 1990. The standard methods for observation and analysis in lake eutrophication, 2nd ed. Environmental Science Press, Beijing (in Chinese). [Google Scholar]
- Lauridsen T.L. and Lodge D.M., 1996. Avoidance by Daphnia magna of fish and macrophytes: chemical cues and predator-mediated use of macrophyte habitat. Limnol. Oceanogr., 41, 794–798. [CrossRef] [Google Scholar]
- Liu W., Hu W., Chen Y., Gu X., Hu Z., Chen Y. and Ji J., 2007. Temporal and spatial variation of aquatic macrophytes in West Taihu Lake. Acta Ecol. Sin., 27, 159–170. (in Chinese with English abstract). [Google Scholar]
- Liu Z.W., Zhong P., Zhang X., Ning J., Larsen S.E. and Jeppesen E., 2014. Successful restoration of a tropical shallow eutrophic lake: strong bottom-up but weak top-down effects recorded. In: Kattel G. (eds). Australia-China wetland network research partnership. Proceedings of the Australia-China Wetland Network Research Partnership Symposium, Nanjing, China, 78–86. [Google Scholar]
- Pípalová I., 2002. Initial impact of low stocking density of grass carp on aquatic macrophytes. Aquat. Bot., 73, 9–18. [CrossRef] [Google Scholar]
- Phillips D. and Gregg J.W., 2003. Source partitioning using stable isotopes: coping with too many sources. Oecologia, 136, 261–269. [CrossRef] [PubMed] [Google Scholar]
- Scheffer M. and Jeppesen E., 1998. Alternative stable states. In: Jeppesen E., Søndergaard M. and Christoffersen K. (eds.). The structuring role of submerged macrophytes in lakes. Springer-Verlag, New York, 397–406. [Google Scholar]
- Zhang L., Li K., Liu Z. andMiddelburg J.J., 2010. Sedimented cyanobacterial detritus as a source of nutrient for submerged macrophytes (Vallisneria spiralis and Elodea nuttallii): an isotope labeling experiment using 15N. Limnol. Oceanogr., 55, 1912–1917. [Google Scholar]
- Zhang M., Yu J., He H., Li K., Chen F., Guan B., Hu Y., Su Y., Du Y. and Liu Z., 2012. Effects of ecological restoration on water quality of Wuli Bay, Lake Taihu. Ecol. Sci., 31: 240–244. (In Chinese with English abstract). [Google Scholar]
Cite this article as: J. Yu,W. Zhen, B. Guan, P. Zhong, E. Jeppesen and Z. Liu, 2016. Dominance of Myriophyllum spicatum in submerged macrophyte communities associated with grass carp. Knowl. Manag. Aquat. Ecosyst., 417, 24.
All Tables
Physical and chemical parameters of the restored part of Lake Qinhu during the sampling months.
All Figures
 |
Fig. 1 Composition and total biomass of submerged macrophytes in the restored part of Lake Qinhu in September and December. Error bars represent the standard deviation (SD) of different sampling sites. |
| In the text | |
 |
Fig. 2 Structure of the fish community in the restored part of Lake Qinhu in September and December, 2011. (A) total fish number per unit effort, NPUE; (B) total fish biomass per unit effort, BPUE; (C) per cent abundance of each fish species; (D) per cent biomass of each fish species. |
| In the text | |
 |
Fig. 3 Stable isotopic signatures of grass carp and macrophytes (A) and the per cent contribution of macrophytes to the carbon source of grass carp (B) estimated using the IsoSource model. Error bars represent the standard deviation (SD) of different individuals. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


