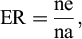| Issue |
Knowl. Manag. Aquat. Ecosyst.
Number 425, 2024
Anthropogenic impact on freshwater habitats, communities and ecosystem functioning
|
|
|---|---|---|
| Article Number | 12 | |
| Number of page(s) | 8 | |
| DOI | https://doi.org/10.1051/kmae/2024010 | |
| Published online | 17 June 2024 | |
Research Paper
Understanding the microplastic pollution impact on Chironomus sancticaroli larvae development and emergence
1
University of São Paulo, Institute of Biosciences, Department of Ecology, Rua do Matão 321, 05508-090, São Paulo, Brazil
2
University of São Paulo, Institute of Chemistry, Department of Fundamental Chemistry, Prof. Lineu Prestes Avenue 748, 05508-000, São Paulo, Brazil
* Corresponding author: queiroz@ib.usp.br
Received:
5
January
2024
Accepted:
16
May
2024
Plastic materials are increasingly present in our lives. It is estimated that more than 360 million tonnes of plastics are produced annually worldwide. Particularly, single-use plastics represent an important segment in plastic production. In this context, plastic contamination, and consequently microplastic release, has become a growing concern for aquatic ecosystems. In this study, we investigated the effects of exposure to polyethylene terephthalate (PET) microplastics (<32 μm) on Chironomus sancticaroli larvae. The larvae were exposed to different concentrations of PET particles (0 (control group), 500, and 5,000 particles.kg−1 of dry sediment) for 10 days. Our results demonstrated that C. sancticaroli larvae displayed PET microplastics in their digestive tracts, and the ingestion increased with increasing PET concentrations. Plastic particles in the digestive tract can reduce the energy obtained by larvae feeding and, consequently, impair their development. The adult emergence rate displayed a significant decrease observed at the highest PET concentration compared with the control group. These findings reinforce existing concerns that microplastics, at concentrations currently found in the natural freshwater environments, can impact the development of benthic macroinvertebrates and, consequently, result in an unbalance in the freshwater ecosystems.
Key words: PET / Diptera / macroinvertebrate / long-term exposure / plastic pollution
© L.D.O. Hallai et al., Published by EDP Sciences 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
1 Introduction
Since the advent of the mass production of plastic materials, the presence of plastic waste in marine and freshwater ecosystems has been documented (Geyer et al., 2017). In recent decades, the dissemination of this waste in the environment has reached alarming proportions, resulting in significant concerns for the health of aquatic ecosystems (Kasavan et al., 2021). The weathering of larger plastics has led to the release of countless particles with a diameter smaller than 5 mm, known as microplastics (Zhang et al, 2021). These plastic particles have spread widely in oceans and freshwater bodies due to their resistance to natural degradation processes (Ashrafy et al., 2023).
Despite plastics being structurally stable and therefore considered inert materials, microplastics pose an environmental concern due to their size, which makes them bioavailable to various organisms in natural environments, with the potential for biomagnification throughout the food chain (Law and Thompson, 2014). In addition to being found in large quantities in the water column and sediments, recent studies have shown that plastic particles are also present in various organs of aquatic organisms, encompassing a wide range of species, such as fish (Zazouli et al., 2022), crustaceans (Yin et al., 2023), and insects (Ribeiro-Brasil et al., 2022). This widespread distribution and accumulation of microplastics in aquatic organisms raise concerns about potential ecological and health impacts. Ecotoxicological evaluation provides important information about the impacts of contaminants on the biota and, for this purpose, different model organisms can be used in these studies.
Dipterans of the Chironomus genus are among the most used organisms as model organisms in assessing the toxicity of various contaminants (Pinto et al., 2021; Rebechi et al., 2021). Chironomus sancticaroli (C. xanthus; C. domizii) has recently been used in Latin America as it is an endemic organism of great importance in the freshwater ecosystem food webs, making it more representative of South American ecosystems (Felipe et al., 2021). Chironomids are often the most abundant macroinvertebrate group distributed in lentic and lotic ecosystems. They play an important link between producers and consumers (Karima, 2021). These organisms live in direct contact with the sediment and can remain buried a few centimeters deep (Viveiros, 2012). The larvae have a non-selective detritivorous feeding behavior, which means they can ingest environmental pollutants, such as plastic particles, along with the sediment while feeding (Palacio-Cortés et al., 2022). Given this context, scientific investigations aiming to elucidate the relationship between microplastics and the success of insect larval emergence are crucial for assessing the potential impacts of these pollutants on the structure and functioning of aquatic ecosystems.
In this context, the present study aimed to investigate the chronic effects of ingestion of irregular PET particles (< 32 μm) on developing C. sancticaroli under environmentally relevant concentrations. Polyethylene terephthalate (PET) occupies the sixth position among the most produced plastic polymers representing approximately 10% of the global production (Geyer et al., 2017) and, for this reason, represents a significant portion of microplastic pollution in freshwater environments.
2 Materials and methods
2.1 Culture and maintenance of Chironomus sancticaroli
The C. sancticaroli larvae used in this study were obtained from a continuous culture maintained at the Laboratory of Limnology of the University of São Paulo. The culture followed the recommendations of Fonseca and Rocha (2004). Briefly, the culture of C. sancticaroli was kept in a plastic tray, covered by nylon mesh support to prevent the escaping of winged individuals. Cultures were maintained under constant aeration using an aquarium air pump (Boyu®, SC-7500). The tray contained a thin layer of fine sand calcined (0.5 cm) as a substrate for larval-stage individuals. Mineral water (Minalba® − pH 7.7, conductivity 143 μS.cm−1) was used as a culture medium. The tray was maintained in a room with a controlled temperature (25 ± 2 °C) and photoperiod (12-12 / light-dark). The feeding consisted of a 40 mL of TetraMin® fish food suspension (5 g L−1) offered 3 times a week. For obtaining larvae for experiments, newly laid eggs were transferred to glass containers containing sediment and mineral water and maintained under the same culture conditions. After hatching, organisms at 5 days of age (second instar) were selected for the experiment.
2.2 PET microplastics
PET microplastics were obtained by grinding a blue-colored bottle of mineral water obtained commercially. The PET bottle was cut into pieces of approximately 2 × 2 cm using metal scissors, and these fragments were subjected to high-energy grinding (Cadence®, MDR302-127) for approximately 15 min. The resulting fragments were passed through a system of metal sieves until particles within the size ranging from 24 to 32 µm were obtained (Fig. 1). This size range was chosen to be similar to the particles present in the sediment and naturally ingested by this species (Viveiros, 2012).
To facilitate the visualization of the microplastics inside the organisms, the particles were stained with the fluorescent dye Nile Red (99% pure, INLAB, Brazil) at a concentration of 300 mg L−1. The stained particles were suspended in ultrapure water, and the concentration was determined by manual counting of 10 µL (n = 3) using a Zeiss Discovery V12 stereomicroscope (10 µL = 16.45 ± 3.15 particles). The solution was kept at 4 °C in the dark until its use.
The shape of the particles was observed and characterized using a Zeiss Discovery V12 stereomicroscope. The chemical composition was determined using Fourier-transform infrared spectroscopy (FTIR). The FTIR spectra were measured using a Bruker Alpha spectrometer in the 400–4000 cm−1 region, with a standard KBr beam splitter and a highly sensitive DLATGS detector. The spectra were recorded using the Attenuated Total Reflection (ATR) module: ATR Platinum, equipped with a diamond crystal as the reflecting element. The spectra were obtained with 128 accumulations and a resolution of 2 cm−1.
 |
Fig. 1 Process of obtaining and visualizing polyethylene terephthalate (PET) microplastics with a size range of 24 to 32 µm. A) The original bottle of mineral water was obtained commercially. B) Plastic from the bottle cut into approximately 2 × 2 cm pieces for grinding. C) Visualization of PET particles under stereo microscopy after grinding. |
2.3 Exposure to PET microplastics
For the chronic exposure assay, three treatment groups were prepared, where the second-instar larvae were exposed to irregular PET particles (24–32 µm) at concentrations of 500 and 5,000 particles kg−1 of dry sediment, referred to as C1 and C2, respectively. A control group with no PET particles was also prepared. The concentrations used were selected based on studies that revealed an average of approximately 129 particles g−1 of microplastics in freshwater sediments (Yin and Zhao, 2023).
Each PET treatment and the control group comprised four replicates (n = 4). Each replicate consisted of a glass vessel with a diameter of 8 cm, containing 60 g of calcined fine sand. In the PET treatments, an aliquot of the PET particle suspension corresponding to the desired concentrations was added to each test vessel. Then, 1 mL of TetraMin® suspension was added to the sand. The control treatment received only the food suspension. The containers were then kept in an oven at 60 °C until completely dried. The drying process was an important step to promote the adhesion of PET particles in the sediment grains avoiding their resuspension when water is added to the test vessel. After completely drying, 340 mL of mineral water, previously aerated for 48 h, was carefully added to avoid resuspension of the PET particles. Subsequently, 25 larvae were added to each replicate. The test vessels were covered with nylon mesh and kept under constant aeration (1 bubble s−1) using an aquarium air pump. The experiment was maintained under the same temperature and photoperiod conditions as the culture. The test vessels were monitored daily for verification of mortality during the exposure period. The larvae were fed every two days with 1 mg of TetraMin® food per organism. Evaporated water was replenished as needed to maintain a volume of 340 mL per test vessel.
On the 11th day of the experiment, larvae from the fourth replicate of each treatment and the control group were collected, and photographed, and their length was measured using ImageJ software. The counting of PET microplastics ingested was carried out under inverted fluorescence microscopy (Leica DMi8). The remaining replicates were maintained for emergence analysis until no live larvae or pupae were found. The emerged adults were collected daily and preserved in 70% ethanol. The adult emergence rate (ER) (OECD, 2023) was calculated as follows:
where:
ER = emergence rate; ne = number of emerged mosquitoes per replicate; na = number of larvae introduced per replicate.
2.4 Statistical analysis
The data obtained in this study were expressed as mean ± standard deviation (SD) or mean percentage (%). The normality of the data was assessed using the Anderson-Darling test (p < 0.05). One-way analysis of variance test (ANOVA) along with Dunnett's test (p < 0.05) was used to compare treatments to the control group from the normal data obtained in the development and emergence analysis. Mann-Whitney test (p < 0.05) was used to compare the ingestion data between treatments. The statistical analyses were performed using MINITAB 14 software.
3 Results
3.1 Characterization of PET microplastics
The PET microplastics displayed irregular morphology and expected size (<32 μm) (Fig. 1C). FTIR (ATR) analysis confirmed the polymer composition, polyethylene terephthalate (PET), as shown in Figure 2. The bands at 1711 and 1239 cm−1 are related to the C=O and C=O stretching of the ester group. Moreover, the bands at the region of 1016 and 1091 cm−1 indicate the 1,4- substitution of the benzene ring and the band at 722 cm−1 is the out-of-plane deformation of the carboxylic substituents on the aromatic ring. The region between 2700 and 3200 cm−1 is related to the aliphatic CH stretching, here we observed at ca. 2960 cm−1 and aromatic CH at ca. 3060 cm−1 (Holland and Hay, 2002).
 |
Fig. 2 FTIR analysis confirms the polymeric composition of PET microplastics. |
3.2 PET microplastic effects on C. sancticaroli
Throughout the experiment, no larval mortality (0.0%) was observed in any treatment groups (control group, C1, and C2). Considering PET ingestion, we observed a significant difference between treatments (Mann-Whitney, p < 0.05). The average ingestion per individual was determined at 0.4 ± 0.7 and 1.64 ± 1.4, for C1 and C2, respectively. In the lower concentration treatment (C1), particles were detected in the digestive tract of 32% of organisms (Fig. 3A). Among the larvae that had particles in their digestive tract in C1, 75% had only one particle and 25% had two particles (Fig. 3A). In the highest concentration (C2), 80% of organisms in the higher concentration treatment (C2) (Fig. 3B). Of these, 45% of larvae had one particle, 30% had two particles, 5% had three particles, 15% had four particles, and 5% had five particles (Fig. 3B). The presence of PET microplastics in the digestive tract of C. sancticaroli was confirmed by the images obtained through fluorescence microscopy (Fig. 4).
After 10 days of the assay, there was no significant difference in the length of larvae between both PET treatments (C1 and C2) and the control group (Dunnett's test, p < 0.05) (Fig. 5A). However, we observed a significant decrease in the emergence rate of C. sancticaroli. At the end of the test, the emergence rate of adults showed a significant difference in the treatment with high PET concentration (C2). The emergence in this treatment was reduced by 22.41% compared to the control group (Dunnett's test, p < 0.05) (Fig. 5B).
 |
Fig. 3 Ingestion of PET microplastics by Chironomus sancticaroli. Percentage of Chironomus sancticaroli individuals that presented particles in the digestive tract after 11 days of exposure to two concentrations of PET: C1: 500 particles.kg−1 (A) and C2: 5000 particles.kg−1 (B). |
 |
Fig. 4 PET microplastics ingested by Chironomus sancticaroli larvae. |
 |
Fig. 5 Length (A) and adult emergence rate (B) of Chironomus sancticaroli larvae (mm) per treatment (Control group, C1: 500 particles.kg−1 dry sediment, and C2: 5000 particles.kg−1 dry sediment). The square symbol (□) represents the mean length of larvae for each treatment. The asterisk (*) indicates a significant difference compared to the control group according to the Dunnett test (p < 0.05). |
4 Discussion
In the present study, we observed the presence of PET microplastics in the digestive tract of C. sancticaroli. Our results suggest that the ingestion and presence of particles in the digestive tract of C. sancticaroli seem to be directly related to the availability of particles in the sediment. At the highest concentration (C2), the incidence of particles was higher in number and frequency compared with the C1 treatment.
Freshwater benthic macroinvertebrates are particularly susceptible to the consumption of microplastics present in the sediment of these environments (Scherer et al., 2017). The ingestion of microplastics by chironomids has already been reported in other studies. In toxicological assays, larvae of C. riparius ingested PS beads (1–90 μm) (Scherer et al., 2017), irregular polyethylene (PE) particles (40–350 μm) (Silva et al., 2019), polyvinyl chloride (PVC) particles (<50 μm) (Scherer et al., 2020), and PET fibers (50 μm) (Setyorini et al., 2021). Studies that also used the species C. sancticaroli observed the ingestion of polypropylene (PP) microplastics (26 μm) (Rani-Borges et al., 2023), polystyrene (24 μm) (Queiroz et al., 2024b), and nylon particles (<50 μm) (Palacio-Cortés et al., 2022). The literature data demonstrate that particles up to 350 μm can be ingested by Chironomus sp. larvae, although particles between 32–63 μm tend to be more consumed (Silva et al., 2019). This information is particularly relevant since microplastics up to 300 μm may constitute the most representative size class in freshwater environments (Alam et al., 2019; Queiroz et al., 2024a). Thus, in contaminated environments, these organisms are constantly exposed to plastic particles that can be ingested resulting in negative effects on different aspects of their life cycle.
Different effects have been observed on chironomids after ingestion of microplastics. These studies have observed changes in the survival rates, growth, and reproduction of chironomids, such as C. riparius (Scherer et al., 2017; Silva et al., 2019) and C. tepperi (Ziajahromi et al., 2018). More recently, larvae of C. sancticaroli have been studied regarding the effects of particle ingestion on survival rates and changes in enzymatic biomarkers (Rani-Borges et al., 2023; Queiroz et al., 2024b) and the composition of the intestinal microbiota (Palacio-Cortés et al., 2022). Changes in growth and emergence of chironomids seem to be related to pseudo-satiation. The sense of pseudo-satiation is promoted by the presence of microplastics in the digestive tract which reduces foraging behaviors (Prata et al., 2023). Once exposed to contaminants, chironomids have energy costs increased to supply energy demand related to their metabolism, such as the detoxification mechanisms (Péry et al., 2003). The impaired obtaining of energy combined with other energetic demands of the organism, such as inflammatory reactions, oxidative stress, or physiological changes, contribute to the negative energy balance (Prata et al., 2023). Thus, if we consider scenarios with higher microplastic concentrations, the inanition of Chironomidae larvae can be even more accentuated.
From an ecological perspective, the ingestion of microplastics is also concerning as C. sancticaroli serves as food for other species (Viveiros, 2012). Thus, the presence of microplastics in the gastrointestinal tract of this species can enhance the transport of particles along the food chains in freshwater ecosystems. Some studies have suggested a trend of bioaccumulation of microplastics in aquatic organisms. However, further field research is needed to corroborate the results of laboratory experiments helping to understand the potential impacts of microplastic bioaccumulation on different species and its ecological implications in freshwater ecosystems (Bhatt and Chauhan, 2022; Neves et al., 2022). Another aspect associated with microplastic ingestion is related to the ability of chironomids to fragment microplastics. It was demonstrated that C. sancticaroli larvae have an important role in the biofragmentation of microplastics. The biofragmentation process causes damage to plastic particles and reduces them through digestive processes resulting in the formation of nanoplastics (Queiroz et al., 2024b).
The influence of size on microplastic toxicity has been reported in the literature. Some studies have suggested that the toxicity of microplastics can be increased as the size of particles decreases. For example, Silva et al. (2019) exposed Chironomus riparius larvae to PE microplastics (40–48 μm) and observed a reduction in larval growth starting from a concentration of 2.5 g kg−1 sediment. Furthermore, the authors observed that the effects caused by larger particles (125 and 350 μm) on larval development only occurred at concentrations of 10 g kg−1 sediment and above. These data suggest that smaller particles may be more toxic. On the other hand, our results related to the larval length did not show a significant decrease even using plastic particles smaller (<32 μm) than the mentioned study. This absence of effects on larvae growth can be attributed to the low concentrations adopted in the present study. Unlike the investigation conducted by Silva et al. (2019), we used concentrations closer to those found in the natural environment to provide more realistic responses occurring at environmentally relevant concentrations.
In the present study, we observed a significant decrease in the emergence rate at the highest PET treatment (C2) compared with the control group. The emergence of C. sancticaroli in the control group was 77%, meeting the OECD (2023) standard of ER > 70%. Other studies investigating the effect of microplastics on the emergence rate of Chironomus spp. have also observed changes in the life cycle of these organisms. Ziajahromi et al. (2018) exposed individuals of Chironomus tepperi to PE particles of different size classes (1 to 126 μm). They observed a reduction in the emergence rate in all treatments, with no significant difference among the size treatments. The emergence of C. riparius was affected after exposure to polyamide (PA) microparticles (<180 μm) at concentrations of 100 and 1000 mg kg−1 (Khosrovyan et al., 2020). On the other hand, at environmentally relevant concentrations (100 mg kg−1 or 10,000 particles kg−1), PA did not cause changes in the emergence of C. riparius (Khosrovyan et al., 2020). Other studies also did not observe significant changes in the emergence of C. riparius after exposure to two different treatments containing tire rubber particles with average sizes of 82 and 3,724 μm (Carrasco-Navarro et al., 2021). Castro et al. (2022) also did not observe significant changes in the emergence of C. sancticaroli exposed to different concentrations of PE (40–48 μm). Relative to the PET polymer, Setyorini et al. (2021) exposed C. riparius to PET fibers of 50 μm at different concentrations (500–50,000 particles kg−1) and demonstrated that particles can be carried through life stages until adulthood. However, no changes in the larval emergence and growth were observed.
It is important to highlight that the difference in the effects of microplastic exposure on the development and emergence of chironomids appears to be associated with the specific toxicity of each microplastic type, which is dependent on the particle size and its concentration in sediment (Silva et al., 2019; Scherer et al., 2020). Furthermore, most studies use microplastics at concentrations higher than those found in natural environments and for a short exposure period (Phuong et al., 2016). Here, we reinforce that we aimed to approach the levels of microplastic pollution currently found in freshwater environments to assess its impact on the life cycle of C. sancticaroli.
From an ecological perspective, microplastic pollution can affect not only individual health but also the associated ecosystems. Considering that benthic macroinvertebrates represent an important group in the degradation of organic matter and food webs of aquatic systems, negative effects on this community may represent an unbalance in the freshwater ecosystems. Moreover, terrestrial ecosystems can also be impacted because chironomid adults are inserted in terrestrial food webs. By examining the life cycle impacts of microplastic pollution on this key species, we shed light on the broader consequences for freshwater ecosystems.
5 Conclusion
The ubiquitous presence of microplastics in freshwater environments can trigger relevant impacts on these ecosystems. This study adopted environmentally relevant concentrations (500 and 5,000 particles kg−1) to bring more realistic information about impacts of microplastic pollution. C. sancticaroli larvae ingested irregular PET microplastics (< 32 µm) demonstrating that ingestion can represent an important exposure pathway to these organisms. The presence of plastic particles in the digestive tract can induce pseudo-satiation in these organisms and, consequently, an energetic imbalance impairing their development. The exposure to these particles resulted in a reduction in the emergence rate of these larvae at the highest concentration tested. These results indicate that PET microplastics, at concentrations currently found in the natural freshwater environments, pose a risk for maintaining chironomid populations. This study constitutes an initial approach to investigating the effects of PET particles on Chironomus sancticaroli larvae. For future studies, sensitive endpoints should be considered to understand the toxic effects at the physiological level that could reflect the changed emergence of insect larvae. Also, generational tests should be developed to comprehend the populational effects.
Funding details
This work was supported by the Pro-rectory of Research and Innovation of the University of São Paulo under Grant PIPAE/2021, and FAPESP under Grants 2021 /10637-2 and 2019/10845-4.
Data availability statement
Data are available from the corresponding author upon reasonable request.
Conflit of Interest
The authors report there are no competing interests to declare.
References
- Alam FC, Sembiring E, Muntalif BS, Suendo V. 2019. Microplastic distribution in surface water and sediment river around slum and industrial area (case study: Ciwalengke River, Majalaya district, Indonesia). Chemosphere 224: 637–645. [CrossRef] [PubMed] [Google Scholar]
- Ashrafy A, Liza AA, Islam MN, Billah MM, Arafat ST, Rahman MM, Rahman SM. 2023. Microplastics pollution: a brief review of its source and abundance in different aquatic ecosystems. J Hazard Mater Adv 9: 100215. [CrossRef] [Google Scholar]
- Bhatt V, Chauhan JS. 2022. Microplastic in freshwater ecosystem: bioaccumulation, trophic transfer, and biomagnification. Environ Sci Pollut Res 30: 9389–9400. [CrossRef] [Google Scholar]
- Carrasco-Navarro V, Muñiz González A-B, Sorvari J, Martínez Guitarte J-L. 2021. Altered gene expression in Chironomus riparius (insecta) in response to tire rubber and polystyrene microplastics. Environ Pollut 285: 117462. [Google Scholar]
- Castro GB, Bernegossi AC, Felipe MC, Ogura AP, de Lima e Silva MR, Corbi JJ. 2022. Polyethylene microplastics and substrate availability can affect emergence responses of the freshwater insect Chironomus sancticaroli. Ecotoxicology 31: 679–688. [CrossRef] [PubMed] [Google Scholar]
- Felipe MC, Bernegossi AC, Cardoso-Silva BN, Dell'Acqua MM, Corbi JJ. 2021. Chironomus sancticaroli generation test applied to chemical contaminants and freshwater sediment samples. Environ Sci Pollut Res 28: 39282–39295. [CrossRef] [PubMed] [Google Scholar]
- Fonseca AL, Rocha O. 2004. Laboratory cultures of the native species Chironomus. Acta Limnol Bras 16: 153–161. [Google Scholar]
- Geyer R, Jambeck JR, Law KL. 2017. Production, use, and fate of all plastics ever made. Sci Adv 3. [CrossRef] [PubMed] [Google Scholar]
- Holland BJ, Hay JN. 2002. The thermal degradation of PET and analogous polyesters measured by thermal analysis-Fourier transform infrared spectroscopy. Polymer (Guildf) 43:1835–1847. [CrossRef] [Google Scholar]
- Karima Z. 2021. Chironomidae: biology, ecology and systematics. The Wonders of Diptera − Characteristics, Diversity, and Significance for the World's Ecosystems, IntechOpen. [Google Scholar]
- Kasavan S, Yusoff S, Rahmat Fakri MF, Siron R. 2021. Plastic pollution in water ecosystems: a bibliometric analysis from 2000 to 2020. J Clean Prod 313: 127946. [CrossRef] [Google Scholar]
- Khosrovyan A, Gabrielyan B, Kahru A. 2020. Ingestion and effects of virgin polyamide microplastics on Chironomus riparius adult larvae and adult zebrafish Danio rerio. Chemosphere 259: 127456. [CrossRef] [PubMed] [Google Scholar]
- Law KL, Thompson RC. 2014. Microplastics in the seas. Science (1979) 345: 144–145. [Google Scholar]
- Neves CV, Gaylarde CC, Baptista Neto JA, Vieira KS, Pierri B, Waite CCC, Scott DC, da Fonseca EM. 2022. The transfer and resulting negative effects of nano- and micro-plastics through the aquatic trophic web—A discreet threat to human health. Water Biol Secur 1: 100080. [CrossRef] [Google Scholar]
- OECD. 2023. Test No. 219: Sediment-water chironomid toxicity using spiked water, OECD. [Google Scholar]
- Palacio-Cortés AM, Horton AA, Newbold L, Spurgeon D, Lahive E, Pereira MG, Grassi MT, Moura MO, Disner GR, Cestari MM, Gweon HS, Navarro-Silva MA. 2022. Accumulation of nylon microplastics and polybrominated diphenyl ethers and effects on gut microbial community of Chironomus sancticaroli. Sci Total Environ 832: 155089. [CrossRef] [PubMed] [Google Scholar]
- Péry ARR, Ducrot V, Mons R, Garric J. 2003. Modelling toxicity and mode of action of chemicals to analyse growth and emergence tests with the midge Chironomus riparius. Aquat Toxicol 65: 281–292. [CrossRef] [PubMed] [Google Scholar]
- Phuong NN, Zalouk-Vergnoux A, Poirier L, Kamari A, Châtel A, Mouneyrac C, Lagarde F. 2016. Is there any consistency between the microplastics found in the field and those used in laboratory experiments? Environ Pollut 211: 111–123. [CrossRef] [PubMed] [Google Scholar]
- Pinto TJ da S, Rocha GS, Moreira RA, Silva LCM da, Yoshii MPC, Goulart BV, Montagner CC, Daam MA, Espindola ELG. 2021. Multi-generational exposure to fipronil, 2,4-D, and their mixtures in Chironomus sancticaroli: Biochemical, individual, and population endpoints. Environ Pollut 283: 117384. [CrossRef] [PubMed] [Google Scholar]
- Prata JC, Silva CJM, Serpa D, Soares AMVM, Gravato C, Patrício Silva AL. 2023. Mechanisms influencing the impact of microplastics on freshwater benthic invertebrates: uptake dynamics and adverse effects on Chironomus riparius. Sci Total Environ 859: 160426. [CrossRef] [PubMed] [Google Scholar]
- Queiroz LG, Pompêo M, de Moraes BR, Ando RA, Rani-Borges B. 2024a. Implications of damming and morphological diversity of microplastics in the sediment from a tropical freshwater reservoir. J Environ Chem Eng 12: 112234. [CrossRef] [Google Scholar]
- Queiroz LG, Prado CCA, Melo EC, Moraes BR, de Oliveira PFM, Ando RA, Paiva TCB, Pompêo M, Rani-Borges B. 2024b. Biofragmentation of Polystyrene Microplastics: A Silent Process Performed by Chironomus sancticaroli Larvae. Environ Sci Technol. [Google Scholar]
- Rani-Borges B, Queiroz LG, Prado CCA, de Moraes BR, Ando RA, de Paiva TCB, Pompêo M. 2023. Biological responses of Chironomus sancticaroli to exposure to naturally aged PP microplastics under realistic concentrations. Ecotoxicology 32: 300–308. [CrossRef] [PubMed] [Google Scholar]
- Rebechi D, Palacio-Cortés AM, Richardi VS, Beltrão T, Vicentini M, Grassi MT, da Silva SB, Alessandre T, Hasenbein S, Connon R, Navarro-Silva MA. 2021. Molecular and biochemical evaluation of effects of malathion, phenanthrene and cadmium on Chironomus sancticaroli (Diptera: Chironomidae) larvae. Ecotoxicol Environ Saf 211: 111953. [CrossRef] [PubMed] [Google Scholar]
- Ribeiro-Brasil DRG, Brasil LS, Veloso GKO, Matos TP de, Lima ES de, Dias-Silva K. 2022. The impacts of plastics on aquatic insects. Sci Total Environ 813: 152436. [CrossRef] [PubMed] [Google Scholar]
- Scherer C, Brennholt N, Reifferscheid G, Wagner M. 2017. Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Sci Rep 7: 17006. [CrossRef] [PubMed] [Google Scholar]
- Scherer C, Wolf R, Völker J, Stock F, Brennhold N, Reifferscheid G, Wagner M. 2020. Toxicity of microplastics and natural particles in the freshwater dipteran Chironomus riparius: Same same but different? Sci Total Environ 711: 134604. [CrossRef] [PubMed] [Google Scholar]
- Setyorini L, Michler-Kozma D, Sures B, Gabel F. 2021. Transfer and effects of PET microfibers in Chironomus riparius. Sci Total Environ 757. [Google Scholar]
- Silva CJM, Silva ALP, Gravato C, Pestana JLT. 2019. Ingestion of small-sized and irregularly shaped polyethylene microplastics affect Chironomus riparius life-history traits. Sci Total Environ 672: 862–868. [CrossRef] [PubMed] [Google Scholar]
- Viveiros W. 2012. Chironomus sancticaroli − do cultivo em laboratório ao ensaio ecotoxicológico com amostras ambientais de sedimento, São Paulo: Universidade de São Paulo. [Google Scholar]
- Yin J, Long Y, Xiao W, Liu D, Tian Q, Li Y, Liu C, Chen L, Pan Y. 2023. Ecotoxicology of microplastics in Daphnia: a review focusing on microplastic properties and multiscale attributes of Daphnia. Ecotoxicol Environ Saf 249: 114433. [CrossRef] [PubMed] [Google Scholar]
- Yin Z, Zhao Y. 2023. Microplastics pollution in freshwater sediments: the pollution status assessment and sustainable management measures. Chemosphere 314: 137727. [CrossRef] [PubMed] [Google Scholar]
- Zazouli M, Nejati H, Hashempour Y, Dehbandi R, Nam VT, Fakhri Y. 2022. Occurrence of microplastics (MPs) in the gastrointestinal tract of fishes: a global systematic review and meta-analysis and meta-regression. Sci Total Environ 815: 152743. [CrossRef] [PubMed] [Google Scholar]
- Zhang K, Hamidian AH, Tubić A, Zhang Y, Fang JKH, Wu C, Lam PKS. 2021. Understanding plastic degradation and microplastic formation in the environment: a review. Environ Pollut 274: 116554. [CrossRef] [PubMed] [Google Scholar]
- Ziajahromi S, Kumar A, Neale PA, Leusch FDL. 2018. Environmentally relevant concentrations of polyethylene microplastics negatively impact the survival, growth and emergence of sediment-dwelling invertebrates. Environ Pollut 236: 425–431. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Hallai LDO, Rani-Borges2 B, Pompêo M, Queiroz LG. 2024. Understanding the microplastic pollution impact on Chironomus sancticaroli larvae development and emergence. Knowl. Manag. Aquat. Ecosyst., 425. 12
All Figures
 |
Fig. 1 Process of obtaining and visualizing polyethylene terephthalate (PET) microplastics with a size range of 24 to 32 µm. A) The original bottle of mineral water was obtained commercially. B) Plastic from the bottle cut into approximately 2 × 2 cm pieces for grinding. C) Visualization of PET particles under stereo microscopy after grinding. |
| In the text | |
 |
Fig. 2 FTIR analysis confirms the polymeric composition of PET microplastics. |
| In the text | |
 |
Fig. 3 Ingestion of PET microplastics by Chironomus sancticaroli. Percentage of Chironomus sancticaroli individuals that presented particles in the digestive tract after 11 days of exposure to two concentrations of PET: C1: 500 particles.kg−1 (A) and C2: 5000 particles.kg−1 (B). |
| In the text | |
 |
Fig. 4 PET microplastics ingested by Chironomus sancticaroli larvae. |
| In the text | |
 |
Fig. 5 Length (A) and adult emergence rate (B) of Chironomus sancticaroli larvae (mm) per treatment (Control group, C1: 500 particles.kg−1 dry sediment, and C2: 5000 particles.kg−1 dry sediment). The square symbol (□) represents the mean length of larvae for each treatment. The asterisk (*) indicates a significant difference compared to the control group according to the Dunnett test (p < 0.05). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.