| Issue |
Knowl. Manag. Aquat. Ecosyst.
Number 426, 2025
Conservation genetics
|
|
|---|---|---|
| Article Number | 20 | |
| Number of page(s) | 19 | |
| DOI | https://doi.org/10.1051/kmae/2025015 | |
| Published online | 01 July 2025 | |
Research Paper
Human impacts on the distribution and genetic diversity of Eurasian minnows (Phoxinus: Leuciscidae) in the Rhenish Massif
Centre for Molecular Biodiversity Research (zmb), Leibniz Institute for the Analysis of Biodiversity Change, Museum Koenig Bonn, Bonn, Germany
* Corresponding authors: n.sternberg@leibniz-lib.de; m.stange@leibniz-lib.de
Received:
18
November
2024
Accepted:
27
May
2025
Introductions of non-native species, a key driver of biodiversity loss, may be underestimated due to cryptic species. In the Rhenish Massif (western Germany), anthropogenic introductions of Eurasian minnows (Phoxinus) caused discrepancies in genetic origin between historical samples from the Ahr and present-day specimens from the adjacent Sieg basin. This study assesses the autochthony of current Phoxinus populations in Ahr and Sieg and reconstructs introduction pathways using cytochrome c oxidase I (COI) barcoding and stakeholder surveys. We find that Ahr populations, free from intentional stocking, are almost exclusively inhabited by Phoxinus clade 5b (P. csikii). In the Sieg, Phoxinus populations exhibit a zonation pattern: clade 5b dominates mountainous regions, while clade 10 (P. phoxinus) is prevalent in foothill regions. Stakeholder surveys revealed substantial historical introductions from the Main/Neckar basin (clades 5b and 10) and Lower Rhine (clade 10), with rarer instances of Rhône (clade 12, P. septimaniae) and Weser (clade 11, P. cf. morella) minnows introduced as contaminants in brown trout (Salmo trutta) stocking. Combined findings indicate clade 5b's autochthony in the Ahr and mountainous Sieg, while clade 10 is suggested to be native to the Sieg's foothill regions, establishing the Sieg basin as a natural contact zone for these clades.
Key words: Anthropogenic introductions / COI barcoding / haplotype network / stakeholder surveys / cryptic species complex
© N. Sternberg et al., Published by EDP Sciences 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
1 Introduction
The introduction of non-native species is a major cause of global biodiversity loss (Cambray, 2003; Clavero and García-Berthou, 2005; Doherty et al., 2016; Linders et al., 2019), especially in freshwater fishes (Bernery et al., 2022; Britton, 2023; Jia et al., 2019; Milardi et al., 2018; Piria et al., 2018). These species can significantly impact native species (Castorani and Hovel, 2015; Hensel et al., 2021) and habitats (Byers et al., 2010; Rodríguez et al., 2003), making early detection crucial (Simberloff et al., 2013). The frequency and impact of introductions may be underestimated due to cryptic species (Carlton, 1996; Collado, 2017), which are morphologically similar but genetically distinct (Bickford et al., 2007). Cryptic species are either pseudo-cryptic due to their unresolved taxonomy (e.g. Feulner et al., 2006), or true cryptic species because of phenotypic plasticity through stabilizing selection (e.g. Martin and Bermingham, 2000). Morais & Reichard (2018) hypothesised that many sudden range expansions and local increases in abundance could be caused by undetected invading cryptic species.
The Eurasian minnows (genus Phoxinus) exemplify a species complex with high cryptic diversity and a history of introductions (Esposito et al., 2024; Garcia-Raventós et al., 2020; Palandačić et al., 2022, 2017). They are ubiquitous, pan-Eurasian leuciscids (Kottelat and Freyhof, 2007; von Siebold, 1863), which inhabit diverse limnic habitats (Frost, 1943; Svirgsden et al., 2018; Tack, 1972, 1941). Phoxinus phoxinus was taxonomically formalised in 1758 (Linnaeus, 1758), with early controversy about its status as a single species datable to the 19th century (Canestrini, 1864; Heckel and Kner, 1858). Nonetheless, all Phoxinus were commonly synonymised as P. phoxinus (e.g. Berg, 1948; Bianco, 2014; Kottelat, 1997). It was only during the last two decades, that the Europe-wide intensification of research on the genus revealed its taxonomic complexity (Bogutskaya and Naseka, 2004; Denys et al., 2020; Kottelat, 2007; Kottelat and Freyhof, 2007; Palandačić et al., 2020, 2017, 2015). Species delineation in the Phoxinus complex has nevertheless proven difficult, largely due to their cryptic morphological characteristics (Bianco, 2014; Collin and Fumagalli, 2015; Palandačić et al., 2017; Ramler et al., 2017). Finally, by including DNA barcoding it was possible to re-evaluate historical morphology-based species descriptions (Palandačić et al., 2017). Since 2017, the majority of taxonomic analyses and the description of new species in Phoxinus was predominantly based on the mitochondrially encoded cytochrome c oxidase I (COI) gene (Bogutskaya et al., 2023; Denys et al., 2020; Palandačić et al., 2024, 2022, 2020, 2015). To date, there are at least 25 valid Phoxinus species in Eurasia (Artaev et al., 2024a, 2024b; Bayçelebi et al., 2024; Berg, 1948; Bogutskaya et al., 2023, 2020; Bogutskaya and Naseka, 2004; Chen, 1988; Denys et al., 2020; Dyldin et al., 2023; Dyldin and Orlov, 2016; Kottelat, 2007; Kottelat and Freyhof, 2007; Kusznierz et al., 2023; Palandačić et al., 2017; Turan et al., 2023; Zhang et al., 2016) and many more so far unassigned genetic clades (Palandačić et al., 2022, 2020). In Germany, three species are recognized as native: P. phoxinus s. str. Linnaeus, 1758 (Lower Rhine, Meuse), P. csikii Hankó, 1922 (Upper Rhine, Danube) and P. marsilii Heckel, 1836 (Odra) (Denys et al., 2020; Freyhof et al., 2023; Palandačić et al., 2022, 2020). A fourth native clade, P. cf. morella Leske, 1774 (basins of Danube, Ems, Weser, Elbe, and Schleswig-Holstein), has already been classified as a National Responsibility Species (Freyhof et al., 2023), but has yet to be validated as a species due to insufficient data (Palandačić et al., 2017).
The distribution of European temperate species established during the last glacial maximum (23,000–18,000 years ago) and subsequent global warming (Bennett, 1997; Hewitt, 2004, 1999), as well as through ancient connections between major basins (Buj et al., 2017). Based on our current knowledge, the distribution of Phoxinus species and clades does not appear to adhere to zoogeographic patterns, often crossing basins and even major European drainage divides (Palandačić et al., 2020, 2017, 2015; Reier et al., 2022a, 2022b). The occurrence of Phoxinus clades outside of their assumed natural range could often be attributed to human causes such as use as live bait angling and stocking throughout Europe (e.g. Garcia-Raventós et al., 2020; Palandačić et al., 2020). Introduction hotspots include the Iberian Peninsula (Corral-Lou et al., 2019; Garcia-Raventós et al., 2020; Suh et al., 2023), the Alps (De Santis et al., 2021; Palandačić et al., 2020; Tiberti et al., 2022), the Scandinavian Mountains (Borgstrøm, 1973; Miró and Ventura, 2015; Museth et al., 2007) and the Rhenish Massif (Behrens-Chapuis et al., 2021; Knebelsberger et al., 2015). These introductions have led to declines in abundance of competitors (Museth et al., 2007; Tiberti et al., 2022) and prey (Miró et al., 2018; Museth et al., 2010) and in the case of amphibians in the Pyrenees even the fragmentation of populations and local extinctions (Miró et al., 2018).
The Rhenish Massif has been the focus of frequent studies of Phoxinus populations, in particular focussing on the Sieg and Ahr basins (e.g. Behrens-Chapuis et al., 2021; Knebelsberger et al., 2015; Kottelat, 2007; Palandačić et al., 2022). The Ahr basin is historically considered to be rich in fish and Phoxinus were among the most widespread and common species (Nitsche and Hein, 1909; Troschel, 1851). Levels of anthropogenic disturbances over the last 150 years were comparably low (v. d. Borne, 1881), hence the Ahr and its tributaries can be considered to have been in a similar good ecological condition as today (Westermann et al., 2011). Similar to the Ahr, the Sieg basin was characterised by ubiquitous and abundant populations of Phoxinus throughout the whole system in the late 19th century (Kirschbaum, 1865; v. d. Borne, 1881; Wittmack, 1875; Wundsch, 1915). Owing to its good condition, the Sieg basin was still considered an important spawning ground for anadromous species, such as Atlantic salmon Salmo salar, European river lamprey Lampetra fluviatilis and the Sea lamprey Petromyzon marinus (v. d. Borne, 1881). Yet at around the same time, severe anthropogenic disturbances, in particular industrial pollution, began to take their toll on the Sieg ecosystem and led to the extinction of local fish communities (Landois et al., 1892; v. d. Borne, 1881; Wundsch, 1915). In the two main rivers of the basin, Sieg and Agger, downstream dilution of contaminants led to the establishment of segregated fish populations, separated by repeatedly emerging new sources of pollution (Wundsch, 1915). Simultaneously, smaller and less strongly influenced tributaries such as the Nister and the Sülz acted as functional biological units (Wundsch, 1915), serving as refuges for undisturbed fish populations (v. d. Borne, 1881). Ongoing pollution until the late 1990s ultimately led to the disappearance of Phoxinus from many streams within their historical range (Klinger, 2001; Landois et al., 1892). In the late 20th century, Phoxinus were considered rare in the Sieg basin (Freyhof, 1998) and the local populations were strengthened by stocking (Borchard et al., 1986). Vast improvements in water quality since the late 1980s were recognisable across the whole Sieg basin, due to innovation and expansion of industrial and municipal sewage treatment plants (Arndt-Dietrich, 2002; Gellert, 2001a, 2001b; Reifenrath and Schmieds, 2001). This was accompanied by growing, self-sustaining Phoxinus populations in the Sieg basin around the turn of the millennium (Klinger, 2001).
Investigations into the genetic identity of historical Phoxinus samples from the Ahr via COI-barcoding revealed predominantly identical or similar haplotypes to recent samples originating from the neo-type locality of P. phoxinus s. str. in the Agger (50°50'N 7°12'E), the major tributary of the Sieg (Knebelsberger et al., 2015). This led to the conclusion that P. phoxinus must be the autochthonous Phoxinus species in the Sieg as well as in the Ahr basins (Knebelsberger et al., 2015). However, the occurrence of Phoxinus clades in the Rhenish Massif derived from present-day samples widely differs from that of historical samples (Knebelsberger et al., 2015; Palandačić et al., 2020). In the Sieg basin alone, three distinct species of Phoxinus, namely P. phoxinus, P. csikii, and P. septimaniae, were identified using DNA barcoding (Behrens-Chapuis et al., 2021; Knebelsberger et al., 2015; Palandačić et al., 2020, 2017). This is taxonomically problematic for the species name P. phoxinus, which is currently associated with “clade 10” (Knebelsberger et al., 2015), as its neotype (NRM-55108; Kottelat, 2007) was designated from an area in which multiple species were previously recorded (Palandačić et al., 2022, 2020). As P. phoxinus was considered the autochthonous species, it was concluded that the Phoxinus populations in the River Sieg were threatened by anthropogenically introduced minnows originating from the Danube (P. csikii) (Knebelsberger et al., 2015). A more recent study suggested the occurrence of a contact zone of P. phoxinus and P. csikii in the Rhine basin (Denys et al., 2020). It was further hypothesised that P. csikii could occur autochthonous in the Sieg (Palandačić et al., 2022). To complicate things further, potential hybridisations, introgressions, and incomplete lineage sorting have been detected among several species and clades of Phoxinus (Palandačić et al., 2022, 2020).
Localising the region of origin of introduced species is crucial for managing introductions (Morais and Reichard, 2018; Simberloff et al., 2013). However, a detailed investigation into the origin of stocking material and the extent of stocking activities is still lacking, potentially affecting previous conclusions about the autochthony of Phoxinus species in the Sieg and Ahr rivers.
Therefore, the aim of this study is to revise the understanding of Phoxinus clades' autochthony in the Sieg and Ahr basins through (1) describing the distribution of Phoxinus COI clades on a basin-wide scale, (2) quantifying stocking practices through stakeholder surveys, and (3) verifying the origin of introduced Phoxinus using a haplotype network.
2 Materials and methods
2.1 Focal river catchments
The 897 km2 large Ahr basin lies in the Ahr hills, an eastern part of the Eifel low mountain range in the Rhenish Massif, around 40 km south of Cologne (Fig. 1). Its eponymous main river is the 83 km long Ahr, a left sided tributary of the Rhine. The Ahr is home to at least 20 species of fish (Schwevers, 1990). North-east of the Ahr lies the 2832 km2 large Sieg basin. It is located between the Süder Upland and Westerwald low mountain ranges in the Rhenish Massif, around 20 km south-east of Cologne (Fig. 2). Its eponymous main river, the 155.2 km long Sieg, is a right sided tributary of the Rhine. Its main tributary is the 74 km long Agger. At least 43 species of fish and two species of lamprey live in the Sieg basin (Freyhof, 1998).
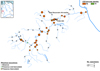 |
Fig. 1 Distribution and COI clade composition of Phoxinus populations in the Ahr basin based on specimens collected in the framework of this study. The red rectangle in the inset shows the location of the Ahr in Germany. Circles represent fished sections: black empty ones with no Phoxinus caught, green empty ones with Phoxinus caught but not barcoded, filled circles for sections with Phoxinus caught and successfully barcoded. The size of the circles is relative to the number of barcoded specimens, the colour denotes the COI-clade identity according to Palandačić et al. (2020). The sampling location of historical specimens caught in the Ahr, first presented in Knebelsberger et al. (2015), is indicated by a black arrow and the years in which they were sampled. |
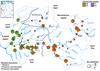 |
Fig. 2 Distribution and COI clade composition of Phoxinus populations in the Sieg basin based on specimens collected in the framework of this study. The red rectangle in the inset shows the location of the Sieg in Germany. Circles represent fished sections: black empty ones with no Phoxinus caught, green empty ones with Phoxinus caught but not barcoded, filled circles for sections with Phoxinus caught and successfully barcoded. The size of the circles is relative to the number of barcoded specimens, the colour denotes the COI-clade identity according to Palandačić et al. (2020). Grey continuous lines indicate the designated Sieg basin zonation into mountainous and foothill region, based on major basin borders (confluences, weirs and artificial waterfalls), administrative districts and geographical regions. Dashed grey lines indicate the sub-region basin divide between Sieg and Agger. All streams discussed in the text are labelled in the figure. The neotype locality of P. phoxinus is indicated by a black target symbol. A single record of clade 11 is indicated by a black arrow. |
2.2 Stakeholder survey
In order to gather information on stocking practices in the Sieg and Ahr basins, stakeholders were reached out to by telephone, email, and post. If available and provided, historical stocking protocols were examined. The stakeholders contacted included fishing rights holders (angling clubs and private individuals), fishing and environmental organisations, former and current local fisheries officers, federal environmental authorities and fish breeders/stocking companies. They were questioned about the natural occurrence of Phoxinus in leased waters; the presence, duration, and extent (i.e. numbers of individuals) of stocking measures; the locations of stocking, and the origin of the parent populations of the stocked Phoxinus. Further introductory pathways (e.g. of anecdotal character) of Phoxinus were also discussed.
2.3 Collection of Phoxinus specimens for barcoding
The aim of the sampling was to cover all available stream types, from brook to main river. Therefore, Ahr and Sieg were sampled across county and federal state boundaries, from krenal regions to their mouths. A total of 58 sampling sites were fished in the Ahr basin between May and June 2022, eighty sampling sites were fished in the Sieg system between May 2022 and October 2023. Phoxinus were captured by electrofishing with a backpack, battery-powered DC electrofishing device (Type EFGI 650, Bretschneider special electronics, Chemnitz and Type IG600, Hans Grassl GmbH, Schönau am Königssee), or with frame nets. On site, specimens were euthanized with clove oil or tricaine mesylate (MS-222), directly after capture. Fin clips were then taken and transferred into molecular grade ethanol (96.6%) and stored at −20 °C until further use. The body of the specimens was preserved in formalin (diluted to 3–7%) for future analysis.
To include potential sources of stocking material informed by the stakeholder interviews additional Phoxinus specimens were collected in France and Germany. These specimens were all euthanized on site in clove oil. In France, samples from the French Rhône, Seine, and Meuse basins were collected in June 2023, in collaboration with the Muséum national d'Histoire naturelle in Paris. French Phoxinus were captured by angling and bottle traps. In agreement with French labour protection laws (substitution of formalin, article r. 4412-66), French Phoxinus were transferred to 75% ethanol, using progressive concentrations over 24 hours for long-term storage. In Germany, local stakeholders, predominantly angling clubs, provided additional samples. They were supplied with the necessary materials (e.g. clove oil, molecular-grade ethanol and 50 ml sample collection tubes). In these cases, the whole fish was placed in molecular grade ethanol and the aforementioned fin clipping was carried out in the laboratory after receipt of the samples.
2.4 Regional classification of the Sieg basin
Based on major basin borders (confluences, weirs, and artificial waterfalls), administrative districts, and geographical regions, the Sieg basin was classified into two key regions, namely the mountainous region and the foothill region (Fig. 2). The mountainous region spreads across two sub-regions: the upper Sieg (from Sieg spring to Sieg waterfall) and the upper Agger (Agger spring to large weirs in Engelskirchen-Ehreshoven; Fig. 2). Accordingly, the foothill region consists of two sub-regions: lower Sieg (Sieg waterfall to Sieg mouth) and the lower Agger (weirs in Ehreshoven to Agger weir in Troisdorf; Fig. 2).
2.5 Phoxinus denominations
Since the taxonomic revision of Phoxinus, using taxonomic characters and single-gene markers (Palandačić et al., 2020, 2017, 2015), valid species and identified genetic clades have been assigned to an ascending numbers scheme. Sensu Palandačić et al. (2017, 2020), P. phoxinus is designated to clade 10, P. csikii to clade 5, P. cf. morella to clade 11, and P. septimaniae to clade 12. P. csikii is divided into two genetic clades, namely 5a (P. csikii s. str.; Danube and Vardar system in the Balkans) and 5b (Alpine and Pre-alpine Danube system, Upper Rhine; Palandačić et al., 2020). For the present geographic focus only P. csikii of clade 5b are relevant. The clades will be referred to as such in the following text, as the information derived from the barcode sequences alone is insufficient for species identification.
Generation and retrieval of, and inference of relationships among COI barcodes from different Phoxinus species in Central Europe.
COI sequences for clade identification (Hebert et al., 2003) were produced in two ways − either directly via Oxford Nanopore Technology (ONT) sequencing or via whole-genome re-sequencing on the Illumina platform. For ONT sequencing the barcoding approach followed that described by Srivathsan et al. (2021). DNA extraction was done with the “HotShot” method (Truett et al., 2000): tissue slices (not more than 10 μg) were placed with 25 μl NaOH/EDTA (25 mM) in a sealed 96-well polymerase chain reaction (PCR) plate and incubated for 30 min at 95 °C, making use of a standard Thermocycler. Then 25 μl neutralisation solution (40 mM Tris-Hcl) was added, the plate sealed again, vortexed and spun down. 1 μl was used for the PCR. PCR was performed immediately or at latest three days after HotShot extraction (which was meanwhile stored at 4 °C). Forward and reverse PCR primers (LCO-1490-JJ: 5'-CHACWAAYCATAAAGATATYGG-3', HCO-2198-JJ: 5'-AWACTTCVGGRTGVCCAAARAATCA-3' (Astrin and Stüben, 2008)) used for amplifying the barcoding fragment of COI were tagged with different 13 bp sequence at 5'-end (see also Srivathsan et al. (2021) for usable tag sequences). Thus, PCR products from different samples could be recognized by their individual combination of forward and reverse tags.
PCR was done in 96-well plates with 20 μl volumes using the Multiplex PCR kit (Qiagen). 10 μl of the Mastermix were mixed with 1 μl of forward and reverse PCR primer, 1 μl DNA from the HotShot extraction, 2 μl Q-solution and 5 μl water. PCR conditions were as follows: Initial degradation was done for 15 min at 95 °C, then 15 cycles with denaturation (35s, 94 °C), annealing (90s, starting at 55 °C, reduced by 1 ° per cycle), and elongation (90s, 72 °C), then 25 cycles with denaturation (35s, 94 °C), annealing (90s, 50 °C), and elongation (90s, 72 °C), followed by a final elongation (10 min, 72 °C).
After PCR, 96 samples were pooled (5μl each) and 160 μl of the pooled mix were used for PCR purification using 0.7× volume of ampure beads and two subsequent washing steps with 80% ethanol. Pools from four plates (each with different combinations of primer tags) were pooled and 48 μl from that mix were used for ONT library construction. For library construction we used the NEBNext companion module for ONT ligation-sequencing (New England Biolabs) for end-repair and adapter ligation, as well as the ligation sequencing kit (SQK-LSK110, Oxford Nanopore Technologies).
In brief 48 μl DNA (pooled PCR products) were used, adding 7 μl Ultra II End-prep reaction buffer and 3 μl Ultra II End-prep enzyme mix. Incubation was done for 5 min at 20 °C and 5 min at 65 °C. After that, DNA was purified by adding AMPure beads (60 μl) and two washing steps with 80% ethanol. The pellet was finally resuspended in 61 μl nuclease-free water. 60 μl from the previous step was used for adapter ligation, adding 5 μl ligation adapter, 25 μl ligation buffer and 10μl T4 ligase. After 30 min incubation at room temperature, the library was purified with AMPure beads (0.9x volume) and finally eluted in 15 μl elution buffer. After quantification of the DNA amount, 100 fmol were used to prepare the library for sequencing (12 μl DNA, 37.5 μl sequencing buffer, 25.5 μl library beads). Each library was sequenced on an already used Minion flowcell after washing them with washing buffer (ONT). The sequencing run lasted 12 hours. ONTbarcoder 2.0 (Srivathsan et al., 2023) was used for demultiplexing sequencing reads (according to the tags) and for elimination of sequencing errors (GenBank identifiers PQ556329 to PQ556558, PQ556560 to PQ556795).
For Illumina sequencing, DNA was isolated using the Qiagen DNeasy® Blood and Tissue extraction kit following the manufacturers instruction for tissues including an RNAse step after lysis. PCR-free libraries were prepared by Novogene GmbH and sequenced on a Novaseq X Plus (PE150) at 15-fold coverage. COI barcodes were retrieved using MitoGeneExtractor v.1.9.5 (Brasseur et al., 2023) (GenBank identifiers PQ556797 to PQ556814, PQ556816 to PQ556819, PQ556821 to PQ556826, PQ556829 to PQ556902, PQ556905, PQ556906, PQ556908 to PQ556913, PQ556917 to PQ557079, PQ557081 to PQ557106, PQ569915).
The newly barcoded specimens were assigned to denominated clades, utilizing already published and designated sequences (Palandačić et al., 2020; Tab. S1) as a reference dataset for our COI sequences. The latter were obtained from NCBI databases (National Center for Biotechnology Information; Sayers et al., 2021). All sequences were aligned using mafft v7.490 with options − retree 1–reorder (Katoh and Standley, 2013) and ordered according sequence identity based on a neighbour joining tree. Sequences from our dataset that clustered to the reference sequences obtained from Table S1 from Palandačić et al. (2020) were assigned to the clade identity of the respective reference barcode.
To understand the geographical distribution of Phoxinus clades across the two basins, Ahr and Sieg, the clade identities derived from 116 successfully sequenced barcodes from specimens from the Ahr and 577 barcodes from Sieg specimens, totalling 693 specimens and barcodes, were mapped to their respective sampling locations (Figs. 1 and 2).
To infer the larger spatial distribution of clades in Europe and whether barcodes from other regions than the middle Rhine cluster with barcodes from Sieg and Ahr a haplotype network was generated. To do so, COI sequences of Phoxinus from previously published studies were retrieved from BOLD (Barcode of Life; Ratnasingham and Hebert, 2007) and NCBI (Sayers et al., 2021) spanning across all basins identified as potential stocking resource populations during the stakeholder survey. Sequences from NCBI were only included if they contained no ambivalent base calls (e.g. Y for either C or T at any positions) and were minimum 550 bases long without gaps (–). All sequences were aligned using mafft v7.490 with options − retree 1–reorder (Katoh and Standley, 2013). The alignment was cropped to a length of 550 nucleotides to eliminate any columns with missing data in any of the included sequences. Seventeen barcodes from Sieg and Ahr did not fulfil these criteria and were excluded from the analysis. Therefore, 676 in this study generated Ahr and Sieg barcodes and 73 barcodes from potential resource populations were combined with 176 previously published barcodes (Appendix 1 in the Online Supplementary Material). A median-joining network (Bandelt et al., 1999) among the COI sequences was built using PopART Version 1.7 (Leigh and Bryant, 2015). The haplotype network represents genetically identical sequences as circles and indicates single-nucleotide differences among sequences as mutational steps between circles of the network.
2.6 Ethical statement and collection permits
Fish samples were caught and collected under the permission of the concerned upper and lower fisheries agencies in accordance with the federal states and country laws on fisheries and environmental protection (Germany: Bundesnaturschutzgesetz (BNatSchG); North Rhine-Westphalia: Landesfischereiverordnung (LFischVO NRW), Landesnaturschutzgesetz (LNG NRW); Rhineland-Palatinate: Landesfischereiordnung (FischGDV RP); Hesse: Hessische Fischereiverordnung (HFischV); Baden-Württemberg: Landesfischereiverordnung (LFischVO BW)). This study did not include experiments on living organisms, therefore, no further permissions from federal animal welfare agencies or ethics commissions were required. Samples in France were collected following the Nagoya protocol (French certificate of compliance TREL2302365S/689).
3 Results
3.1 Possible sources of Phoxinus introduction in Ahr and Sieg
The survey of 23 local Ahr stakeholders revealed no records or verbal knowledge of Phoxinus stocking. In particular, none of the twelve surveyed stocking companies could provide any records of Phoxinus stocking in the Ahr basin. The stocking of brown trout and Atlantic salmon was the only common practice across the majority of the basin.
In contrast, the survey of 67 stakeholders across the Sieg basin reported 209 incidents of intentional stocking with Phoxinus between 1981 and 2022 (for a detailed breakdown see Appendices 2 and 3 in the Online Supplementary Material). These introductions primarily served to reintroduce, establish and enhance local Phoxinus populations. Phoxinus stocked in the mountainous region originated from the Ruhr, Kinzig (Main), Dill (Lahn-Middle Rhine), and the Sieg basin itself and were commonly only introduced a single time (Tab. 1). In the foothill region, region-wide stocking was conducted with Phoxinus originating from Franconia (possibly, Main, Neckar (both Rhine), or Danube) and North-Rhine Westphalia (Sieg, Ruhr, or Lippe), while Phoxinus from the Ruhr were also introduced locally (Tab. 1). Despite the wide-ranging stocking efforts, a few streams were free of any intentional stocking. This included the main river Sieg and smaller tributaries like the Werthenbach, Dreisbach, upper Ferndorf, Daadenbach, Elbbach, Wisserbach and Holper Bach in the mountainous region, as well as the Sülz system, the Holper Bach and the Lauterbach in the foothill region (Appendices 2 and 3 in the Online Supplementary Material).
Stakeholders further reported potential unintentional introductions through brown trout stocking contaminated with Phoxinus. Source populations for stocked brown trout were located in the Rhine, Weser, and French river basins. Furthermore, Phoxinus were possibly introduced by anglers, who used them as live bait. Phoxinus for live bait angling were supplied bucket-wise from all over Germany. This practice was only reported in the upper Agger region, but cannot be ruled out for the rest of the Sieg basin.
Summary of stocking practices conducted in the Sieg basin. For a detailed description of the stocking efforts, please see Appendices 2 and 3 in the Online Supplementary Material.
3.2 Phoxinus clade distribution in Sieg and Ahr
Of the 116 successfully barcoded specimens from the Ahr basin, 113 carried a clade 5b COI signature (Fig. 1). They were distributed across the entire Ahr basin from epirhithral (upper stream region equivalent to upper trout region) to epipotamal (lower stream region equivalent to barbel region) regions, including its mouth. Three individuals were assigned to clade 10 according to their barcode and were found randomly distributed across the basin (Fig. 1). Phoxinus were generally missing in krenal (source) regions and small trickles (Fig. 1).
In the Sieg basin, Phoxinus occurred from epirhithral to epipotamal regions (Fig. 2) and were generally absent from the krenal regions, and missing in the close vicinity to the Sieg mouth. Compared to the Ahr, the clade composition of Phoxinus populations was more diverse.
Of the 585 barcoded Phoxinus, 335 were assigned to clade 5b. In the mountainous region, clade 5b was prevalent and occurred exclusively in many populations of the main Sieg river and in particular smaller tributaries. Proportions of clade 5b in the foothill region differed between lower Sieg and lower Agger. In the lower Sieg, clade 5b proportions followed a downstream gradient from prevalence in the upper parts of the Sieg down to near absence at the confluence with the Agger (Fig. 2). Clade 5b was extremely rare and generally absent from the lower Agger region.
Further 223 Phoxinus specimens were assigned to Clade 10. Clade 10 was rare in the mountainous region and only occurred locally. Contrastingly, clade 10 prevailed in the foothill region and occurred exclusively in populations of the Sülz, Naafbach, and the Agger (Fig. 2).
Sixteen specimens of clade 12 were recorded, they occurred randomly dispersed throughout the Sieg basin. The distributional focus of clade 12 was located in the lower Agger, including a site in close proximity to the neotype locality of P. phoxinus (Fig. 2). A single specimen with a clade 11 haplotype was recorded in the Sieg close to the confluence with the Bröl (Fig. 2). The Wahnbach system was identified as the only larger tributary completely free of any Phoxinus in the whole Sieg basin.
3.3 Clustering of mitochondrial COI haplotypes from different European basins
The haplotype network based on COI sequences from NCBI, BOLD, and newly generated sequences (Appendix 1) in this study revealed 55 unique haplotypes that formed eight haplogroups (Fig. 3a). These haplogroups represent the previously described clades and species (sensu Denys et al., 2020; Palandačić et al., 2020): clade 5b (P. csikii), clade 9 (P. marsilii), clade 10 (P. phoxinus), clade 12 (P. septimaniae), clade 13 (P. bigerri), clade 16 (P. fayollarum), clade 23 (P. dragarum) and the not yet taxonomically formalised clade 11 (P. cf. morella).
The large majority of Ahr specimens clustered to clade 5b, sharing haplotypes with Phoxinus from the Rhine (Upper Rhine, Main, Sieg), as well as very few specimens from the Rhône and Meuse (Fig. 3d). Three of the recorded clade 5b haplotypes were unique to the Ahr basin. Ahr specimen that clustered to clade 10 exhibited the same and most common clade 10 haplotype (haplotype 6, Fig. 3c).
Regarding Phoxinus from the Sieg basin, the majority of specimens, predominantly from the mountainous region, clustered to clade 5b (Fig. 3d). Clade 5b haplotypes from the Sieg were shared with Phoxinus from the Rhine (Upper Rhine, Main, Ahr), Meuse, and Rhône. With the exception of haplotypes 5 and 10, there were no shared haplotypes between specimens from the Sieg and the Danube (Fig. 3d). Six recorded clade 5b haplotypes were unique to the Sieg basin.
A little less than half of all Sieg Phoxinus, predominantly from the foothill region, clustered to clade 10, commonly sharing haplotypes with Phoxinus populations in the Rhine (Lower Rhine and Kinzig (Main)), and Meuse (Fig. 3c). A single haplotype was shared between Phoxinus from the Sieg and a single Danube individual (haplotype 114, Fig. 3c). Two of the recorded clade 10 haplotypes were unique to the Sieg basin. Phoxinus from the Seine (haplotype 139) and Normandy (haplotype 181) were at least seven mutational steps distant to the rest of sequences belonging to clade 10 (Fig. 3c).
A small proportion of Phoxinus from the Sieg basin clustered under clade 12, sharing a haplotype with others predominantly from the Rhône (haplotype 22, Fig. 3e). Furthermore, a single Phoxinus from the Sieg basin clustered under clade 12, sharing the most common clade 12 haplotype with others from the Ijssel, Weser, Elbe, and Danube catchments (haplotype 1, Fig. 3a).
No Phoxinus sequences from Sieg or Ahr clustered with sequences of clade 9 (Danube, Lake Neusiedl, Odra and Vistula), clade 13 (Adoure), clade 16 (Grand-Ouest and Rhône) or clade 23 (Gironde, Fig. 3a).
 |
Fig. 3 Clustering of mitochondrial cytochrome oxidase I (COI) haplotypes from different European basins. a. Haplotype network based on 925 COI fragments from Phoxinus specimens from various European freshwater basins (colour-coded) of potential introductory resource populations. b. Overview of the European freshwater basins from which haplotypes were included in the haplotype network. The separate haplotype networks for c. clade 10, d. clade 5b, and e. clade 12 include a further breakdown of investigated basins. Numbers identify relevant haplotypes discussed in the results and discussion sections. Clades according to Denys et al. (2020) and Palandačić et al. (2020): clade 5b (P. csikii), clade 9 (P. marsilii), clade 10 (P. phoxinus), clade 12 (P. septimaniae), clade 13 (P. bigerri), clade 16 (P. fayollarum), clade 23 (P. dragarum), clade 11 (P. cf. morella). |
4 Discussion
Phoxinus in Sieg and Ahr occurred from the epirhithral to the epipotamal regions, but were absent in the actual krenal regions (Figs. 1 and 2). This corresponds to their known distributional range in the Rhenish Massif (Steinberg and NZO, 1992; Tack, 1972, 1941). In the much larger Sieg basin, Phoxinus were also absent from wider and deeper regions closer to the Sieg mouth (Fig. 2), which do not exist in the Ahr in a comparable way. Phoxinus are generally considered to be riffle-dwelling species that prefer fast-flowing, colder water (Blanck et al., 2007). This is why they are absent from slower-flowing lowland streams (Tack, 1941), like the waters close to the Sieg mouth (Fig. 3).
4.1 Autochthony of Phoxinus in the Ahr
Knebelsberger et al. (2015) analysed historical museum samples of Phoxinus from the Ahr collected in 1888 and 1905 (Fig. 1) and identified them to be of clade 10 origin. Clade 10 was subsequently described as autochthonous in the Ahr basin (Knebelsberger et al., 2015). Phoxinus populations in the Ahr basin were never threatened by heavy pollution (v. d. Borne, 1881) and always abundant (Klinger, 2001; Troschel, 1851; v. d. Borne, 1881), therefore stakeholders reported no stocking of Phoxinus at all. Phoxinus populations in the Ahr were consequently believed to be untouched, natural populations of clade 10. Therefore, it came as a surprise that the overwhelming majority of Phoxinus in the Ahr basin was not of clade 10 origin, but rather clade 5b. Clade 5b was system-wide distributed and exclusively, or at least overwhelmingly prevalent in all Ahr populations (Fig. 1). Phoxinus of clade 5b in the Ahr basin further exhibited a high diversity of haplotypes, some of which were unique to the basin (Fig. 3d). The lack of stocking measures, the wide distribution, and prevalence in the entire basin in conjunction with the high haplotype diversity make it very unlikely that clade 5b was introduced into the Ahr basin. Taken together, these features identify clade 5b as the autochthonous Phoxinus in the Ahr basin.
In contrast, Ahr Phoxinus assigned to clade 10 were characterised by only a single, the overall most frequent haplotype of clade 10 (Fig. 3d, haplotype 6), mostly found in the lower Rhine (including the Sieg) and Meuse (Fig. 3c). The rarity and random distribution of clade 10 in the Ahr basin further indicates a more recent introduction, rather than an autochthony in the basin. This may be related to the ornamental fish trade of Phoxinus for aquariums and ponds throughout Europe (Maceda-Veiga et al., 2013; Tack, 1941). Escapes of fish from garden ponds and fish farms are a common phenomenon during flood events (Casimiro et al., 2018; Chan et al., 2019; Raj et al., 2021), which occurred in the Ahr valley in 2016 and 2021. In the course of both severe flood events, residential areas in the Ahr valley were extensively flooded (Birkmann et al., 2023; Mohr et al., 2023). Therefore, Phoxinus of clade 10 haplotypes in the Ahr could be the successors of escapees from captivity. Accordingly, an earlier investigation of the haplotypes of Phoxinus sold in a pet shop neighbouring the Ahr valley (Walter, 2013) revealed minnows of clade 10 (NCBI identifiers KM286863-866; Knebelsberger et al., 2015). This is further supported by the rarity and localisation of clade 10 minnows, which were caught exclusively in the vicinity of human settlements. While stakeholders refrained from actively stocking Phoxinus, stocking of brown trout was ubiquitous in the middle and lower Ahr. Accidental fish stocking with contaminated stocking material is a common route of introduction for fish (Benson, 1999; Gozlan et al., 2010; Rahel, 2004) and has been previously reported in relation to brown trout stocking in Norwegian lakes (Borgstrøm, 1973). An accidental introduction of clade 10 through stocking with brown trout could therefore not be completely ruled out in the Ahr. Overall, these findings characterise clade 10 as allochthonous in the Ahr basin.
These results are in clear contrast to the previously assumed autochthony of clade 10 in the Ahr (Knebelsberger et al., 2015; Kottelat and Freyhof, 2007). Therefore, the present assessment of clade 5b as autochthonous necessitates a comprehensive examination of historical Phoxinus samples from the Ahr beyond barcodes. In particular, an investigation into their genomes should help to clarify the actual species identity of the historical museum samples that had been assigned to clade 10. This is now feasible with advances in museomics (Fong et al., 2023), extractions from formalin-fixed specimens (Hahn et al., 2022), and the recently published Phoxinus reference genome (Oriowo et al., 2025).
4.2 Autochthony of Phoxinus in the Sieg
4.2.1 No minnows of Danube origin in the Sieg river system
Previous research found Phoxinus haplotypes similar to those of historical museum samples from the Ahr (identified as clade 10) at the neotype locality of P. phoxinus in the Agger (Knebelsberger et al., 2015). Due to the relative geographical proximity of Ahr and Agger, it was concluded that Phoxinus clade 10 is also autochthonous in the entire Sieg basin (Knebelsberger et al., 2015). Furthermore, Knebelsberger et al. (2015) attested Phoxinus clade 5b a clear biogeographical distribution, limited to the Danube basin in Southern Germany. Based on reports on stocking efforts, they hypothesised that the autochthonous clade 10 populations in the Sieg basin were threatened by introduced clade 5b Phoxinus from the Danube. The results of the haplotype network contradict this hypothesis (Fig. 3): samples from the Sieg assigned to clade 5b predominantly shared haplotypes with samples from the Rhine, Rhône, and Lake Neusiedl (Fig. 3d). The sharing of haplotypes between the entire Rhine and the Danube was very rare, as evident in the sharing of only two out of 26 clade 5b haplotypes (haplotype 5 and 10, Fig. 3d). Similar observations had been made in previous studies (Esposito et al., 2024; Suh et al., 2023). The lack of haplotype sharing within clade 5b between the Sieg and Danube indicates that there were no intentional, large-scale introductions of Phoxinus from the Danube basin into the Sieg. Hence, Phoxinus populations in the Sieg basin are not threatened by congeneric introductions from the Danube.
Nonetheless, our stakeholder surveys verified that the Sieg basin had indeed been stocked with Phoxinus originating from Southern Germany (Tab. 1, Appendices 2 and 3 in the Online Supplementary Material). For the initial large-scale stocking in the foothill region (1981–1984), Phoxinus were supplied by a company located in Franconia in Southern Germany (Tab. 1). Accordingly, several 5b haplotypes were shared between the Sieg and southern German Phoxinus populations from the upper Rhine and Main basin (Fig. 3d). The initially stocked Phoxinus in the foothill region were thus of Rhine origin, most likely originated from tributaries of the Main or Neckar systems in the close vicinity of the Franconian stocking company.
It seems likely that the sharing of clade 5b haplotypes between the Rhine and Danube, namely haplotype 5 and 10 (Fig. 3d), is not a natural phenomenon. Haplotype 5 was predominantly recorded in specimens from the Danube and was registered in only a single specimen from the Upper Rhine (Fig. 3d). All other Upper Rhine samples exclusively clustered with other clade 5b Phoxinus from the Rhine basin (Fig. 3d). Therefore, it seems more likely that haplotype 5 was anthropogenically introduced in the Upper Rhine population, likely through live bait fishing, which was a common practice in Southern Germany in the 20th century (Tack, 1941). In contrast, haplotype 10 was recorded once in the Danube but was otherwise exclusive to populations in the mountainous region of the Sieg basin (Fig. 3d). Due to this unbalanced distribution, it seems most likely that haplotype 10 was introduced into the Danube basin. This introduction is probably due to the national and international ornamental fish trade with Phoxinus emanating from the Sauerland (Tack, 1941), to which parts of the mountainous region belong. Similar introductions of Phoxinus from the Rhine basin into the Danube basin had previously been reported from Austria (Palandačić et al., 2020), evidenced in the record of a clade 10 haplotype in the Danube basin (haplotype 114, Fig. 3c). However, it cannot be completely ruled out that the occurrence of haplotype 10 in the mountainous region is due to an anthropogenic introduction from the Danube basin. In particular live bait fishing, a common practice in the upper Sieg (Wundsch, 1915) also reported by stakeholders in the upper Agger, could be considered as an introduction route.
In conclusion, all cases of haplotype sharing between the Rhine and Danube basins occurred in regions of intense human activities with Phoxinus. They are thus likely linked to stocking, ornamental fish trade, or live bait fishing, which are among the most common paths of fish introductions (e.g. Gozlan et al., 2010; McEachran et al., 2022; Rahel, 2004; Webb, 2007).
4.2.2 Mountainous region − clade 5b natural, clade 10 introduced by stocking
Phoxinus clade 5b was distributed system-wide and generally prevalent in almost all populations of the mountainous region (Fig. 2). Assuming the autochthony of clade 10 in the entire Sieg basin (Knebelsberger et al., 2015), this would indicate mountainous region-wide stocking efforts with Phoxinus of clade 5b origin. However, with the exception of an introduction of Phoxinus from the Sieg above Siegen, inhabited exclusively by clade 5b (Fig. 2), stakeholders reported no stocking with Phoxinus from populations located in the presumed distributional range of clade 5b (Tab. 1, Appendices 2 and 3 in the Online Supplementary Material). Therefore, the occurrence of clade 5b in the mountainous region should be of natural origin. This hypothesis is supported by the fact that Phoxinus populations not subjected to stocking efforts and distantly removed from stocking locations were characterised by the sole occurrence of clade 5b Phoxinus. This was particularly evident in the upper reaches of the Sieg upstream of Siegen, and smaller, more isolated streams like the Werthenbach, Dreisbach, Daadenbach, Elbbach, and Holper Bach (Fig. 2). The relative seclusiveness of these streams from the heavy pollution that affected the Sieg basin between the late 19th century and the 1980s (v. d. Borne, 1881; Westermann et al., 2011; Wundsch, 1915), enabled the survival of thriving Phoxinus populations (Kirschbaum, 1865; NZO and IFÖ, 2007; v. d. Borne, 1881). Consistently, Phoxinus populations in the mountainous region of the Sieg basin showed a high diversity of haplotypes, some exclusive to the region (Fig. 3d). Our findings thus confirm the hypothesis by Palandačić et al. (2022), that Phoxinus clade 5b is autochthonous in the Sieg basin. We conclude that its natural distribution extends at least across the whole mountainous region, including the Sieg and all its tributaries from its source down to the Sieg waterfall at Schladern and the Agger from its source to the weirs in Ehreshoven.
In contrast, Phoxinus clade 10 was generally less frequent in the mountainous region and its occurrence could always be linked to stocking practices originating from areas that are known to be populated by clade 10: Ruhr, Lippe (both Lower Rhine), and Kinzig (Main) (Fig. 3c), and mixed populations in the Dill (Appendix 2). Consistently, Phoxinus clade 10 occurred exclusively in the sphere of influence of reported stocking efforts (Tab. 1), predominantly in the core areas and downstream of reported introduction sites in the Littfe, Ferndorf, Alche, Sieg below Siegen, Nister, Kleine Nister, and Agger below Gummersbach (Fig. 2). This limited, primarily downstream directed dispersal after stocking is likely linked to the rather sedentary lifestyle of Phoxinus (Balon et al., 1986; Cornelius, 1865; Tack, 1941) and their tendency for downstream dispersal (Bless, 1992; Jones et al., 2021; Popp and Lehr, 2008). Nonetheless, our findings of clade 10 in unstocked and predominantly 5b populations upstream of stocking sites such as in the upper part of the Nister, the Wisserbach, and the Seßmarbach (Fig. 2), also support previous findings of upstream migrations over short distances (Frost, 1943; Jansen et al., 1999: Svirgsden et al., 2018).
The sampled Wiehl population in the upper Agger represents a case where the occurrence of clade 10 is of uncertain origin. While stakeholders reported a county-wide stocking programme in the upper Agger (including the Wiehl) with Phoxinus of clade 10 origin from the Ruhr (Tab. 1), introductions could only be verified for the main stream Agger downstream of Gummersbach. Stakeholders reported the use of Phoxinus as live bait in the upper Agger region, including the Wiehl, which had a long lasting tradition in the mountainous region in general (Wundsch, 1915). Since local Phoxinus populations in the upper Agger region were largely missing in the late 20th century (Borchard et al., 1986; Klinger, 2001; Steinberg and NZO, 1992), the use of Phoxinus from other basins as live bait is very likely. Likely sourced were the neighbouring Lenne and its tributaries, a part of the Ruhr basin, which harboured abundant Phoxinus populations (Tack, 1972) belonging to clade 10 (Fig. 3c; Palandačić et al., 2022).
Hence, we conclude that Phoxinus clade 10 is allochthonous in the mountainous region of the Sieg basin and its occurrence is the result of anthropogenic introductions.
4.2.3 Foothill region − clade 10 natural, 5b introduced by stocking
In contrast to the mountainous regions, it is more difficult to draw conclusions about the autochthony of the Phoxinus clades occurring in the foothill region, as most populations were influenced by stocking measures. The establishment of mixed populations in the foothill region can be traced back to stocking with Phoxinus of clade 5b from the Main and/or Neckar and clade 10 from the Ruhr, Lippe, and/or Sieg (Tab. 1). This led to the formation of a large 5b and 10 mixing zone, which had previously been reported for the entire Sieg basin (Knebelsberger et al., 2015), but is actually located in the foothill region, predominantly in the lower Sieg region, of the Sieg basin (Fig. 2).
Nonetheless, Phoxinus clade 10 was overall most prevalent and occurred in all investigated populations in the foothill region (Fig. 2). Special attention should be paid to the Sülz system, which was only ever moderately polluted (Arndt-Dietrich, 2002; v. d. Borne, 1881; Wundsch, 1915) and historically inhabited by abundant or even mass Phoxinus populations (Borchard et al., 1986). Since stakeholders reported no intentional stocking with any Phoxinus, we consider the Sülz populations as natural. Similar to the Ahr, the Phoxinus clade autochthonous in the Sülz should thus be distributed across the whole system, occurring exclusively or prevailingly. While two clades of Phoxinus, clade 10 and clade 12, occurred in the Sülz (Fig. 2), this was only the case for clade 10. Furthermore, Phoxinus clade 10 occurred in populations of the foothill region that were only ever stocked with clade 5b (Tab. 1, Appendices 2 and 3 in the Online Supplementary Material), namely in the Eipbach, Waldbröl, Homburger Bröl, Lauterbach (as part of the Pleisbach system), and Naafbach. Due to the sedentary lifestyle to limited dispersal ability of Phoxinus species (Balon et al., 1986; Cornelius, 1865; Jansen et al., 1999; Tack, 1941), the occurence of clade 10 in these populations through long-range upstream migration seems unlikely and should be natural. In combination with the identification of haplotypes exclusive to the foothill region, these results identify clade 10 as autochthonous in the foothill region of the Sieg basin.
The autochthony of clade 10 in the foothill region justifies the selection of the neotype locality of P. phoxinus in the Agger (Kottelat, 2007). However, local populations near and at the neotype locality were of mixed clade composition (Fig. 2). Since Phoxinus clades are known to hybridise (Palandačić et al., 2017), the species origin of the specimen has to be verified through genetic analyses and, if necessary, a re-evaluation of its type status should be considered.
The fact that most occurrences of clade 5b in the foothill region were linkable to stocking does not necessarily mean that clade 5b is allochthonous across the entire foothill region. While clade 5b is evidently allochthonous to the lower Agger region, indicated by the absence of clade 5b in almost all lower Agger populations (Fig. 2), it could be autochthonous to the lower Sieg region. This hypothesis is supported by the sharing of clade 5b haplotypes between Phoxinus from the lower Sieg with autochthonous populations from the mountainous region. Several of these haplotypes were exclusive to the Sieg basin, in one case even the lower Sieg region itself (Fig. 3d). Nonetheless, the observed downstream gradient in haplotype composition of Phoxinus populations in the lower Sieg region indicates that this autochthony does not range across the whole region. The shift from clade 5b dominance in the upper parts of the lower Sieg to clade 10 dominance close to the confluence with the Agger (Fig. 2) indicates the edge of the distribution of clade 5b within the Sieg basin. A natural contact zone in the distribution of clade 5b and 10 was likely located somewhere around the mouth of the Eipbach, but is today not identifiable due to the wide-ranging stocking efforts.
4.2.4 Clade 12 occurrence in the Sieg
Clade 12 occurred in intentionally stocked and unstocked Phoxinus populations throughout the Sieg basin. However, the rarity, random distribution and low diversity of clade 12 haplotypes distinguishes clade 12 from clade 5b and clade 10 as allochthonous in the Sieg basin. The natural distribution range of clade 12 spreads across the French Mediterranean catchments, including the Rhône (Corral-Lou et al., 2019; Denys et al., 2020; Kottelat and Freyhof, 2007). Stakeholder interviews did not reveal intentional stocking of Phoxinus from French waters, but introductions of brown trout from France were reported. Unintentional stocking of fish species with contaminated stocking material is a common pathway of fish introductions (Benson, 1999; Gozlan et al., 2010; Rahel, 2004). Cases of Phoxinus introductions associated with brown trout stocking have previously been observed in Norwegian lakes (Borgstrøm, 1973), therefore, a potential pathway of introduction for clade 12 could be stocking of French brown trout fry contaminated with Phoxinus. Phoxinus of clade 12 recorded in the Sieg basin shared their haplotype predominantly with Phoxinus from the Rhône. Due to its geographical proximity, it seems most likely that the introduced Phoxinus originated from the Saône system, the northern part of the Rhône basin. Due to haplotype sharing between Sieg and Meuse populations (Fig. 3c), we can not exclude introductions from French Meuse populations through contaminated brown trout fry. There were no introductions from other French tributaries of the Bay of Biscay (clade 13, 16 and 23) and the English Channel (clade 10 from Seine and Normandy basins) into the Sieg basin, as evident by a lack of any shared haplotypes (Fig. 3a, Fig. 3c).
4.2.5 Clade 11 occurrence in the Sieg
Only the three above discussed clades (5b, 10, 12) of Phoxinus were previously recorded in the Sieg basin (Knebelsberger et al., 2015; Palandačić et al., 2017, 2020, 2022). The identification of a single specimen with a clade 11 haplotype in the river Sieg in the foothill region (black arrow, Fig. 2) is therefore the first record of a fourth Phoxinus clade in the Sieg basin. Clade 11 is generally attributed to the Ems, Weser, and Elbe (Knebelsberger et al., 2015; Palandačić et al., 2020). Stakeholder interviews did not reveal intentional introductions of Phoxinus from these drainages, but revealed that a company located in the Weser basin was responsible for stocking of brown trout in the foothill region for over a decade. Concordant with the introductory pathways of clade 12, the introduction of clade 11 into the Sieg basin is therefore likely linked to contaminated stocking material of brown trout. Combined with its rarity, this identifies clade 11 as allochthonous in the Sieg basin. It can further be ruled out that stakeholders in the Sieg basin were ever supplied with Phoxinus acquired from Poland or Czechia as no other specimen with haplotypes of clade 11 as well as clade 9 (Odra and Vistula) were recorded in this genetic screening of nearly 700 Sieg specimens.
4.3 Natural distribution of Phoxinus clades along the Rhine
In contrast to the here described autochthony of clade 5b in the Ahr (Middle Rhine) and mountainous region of the Sieg basin (Lower Rhine), the natural range of clade 5b had previously been described to be restricted to the Danube and Upper Rhine basin (Denys et al., 2020; Palandačić et al., 2020). A natural occurrence in the Middle Rhine had so far only been hypothesised (Denys and Manne, 2019), and was confirmed through the designation of clade 5b as autochthonous in the Ahr in this study. The description of the autochthony of clade 5b in the Sieg further extends the natural range to the southernmost part of the Lower Rhine. Almost all other Rhine Phoxinus samples from tributaries upstream of the Sieg (Ahr, Main and Upper Rhine) clustered with clade 5b. This indicates that the actual natural range of clade 5b in the Rhine extends across the Upper Rhine and Middle Rhine down to the Sieg in the Lower Rhine.
Phoxinus clade 10 had previously been allocated to the entire Rhine basin (Denys et al., 2020; Kottelat and Freyhof, 2007; Palandačić et al., 2017), yet haplotypes of clade 10 from the Rhine (previous and this study) almost exclusively originated from tributaries of the lower Rhine (Fig. 3c), namely the Sieg, Ruhr, and Lippe. The only other records of clade 10 haplotypes from the Rhine basin originated from the Kinzig and Sinn, tributaries of the Main located in the Spessart low-mountain range in the Upper Rhine basin, far from any other clade 10 populations. Since several supra-regional stocking companies are active in the Spessart region, its occurrence is likely linked to anthropogenic introductions. This is further supported by the occurrence of haplotype 114 in the Kinzig (Fig. 3c), which was also recorded in the Sieg and had been introduced in the Austrian parts of the Danube (Palandačić et al., 2020). Consequently, we conclude that the natural distribution of clade 10 in the Rhine is limited to the Lower Rhine.
These findings confirm a previously hypothesised contact zone of clade 5b and clade 10 in the Rhine region (Denys et al., 2020), which is evidently located in the Sieg basin. According to the concluded natural distribution areas of clades 10 and 5b, contact zones should exist in the upper parts of the lower Sieg and lower Agger. However, their natural occurrence is concealed by the extensive stocking measures.
4.4 Ecological species sorting in Phoxinus
Clade composition of Phoxinus populations across the whole Sieg basin followed a downstream gradient, shifting from prevalence of clade 5b in the mountainous region to clade 10 in the foothill region (Fig. 2). Longitudinal downstream gradients in the occurrence and abundance of Phoxinus have previously been reported from the Danube and Seine basins (Balon et al., 1986; Oberdorff et al., 1993), yet this is the first report of a gradient in clade composition. Since the occurrence of both clades seems to be the result of a natural contact zone in their distributional range, the downstream gradient in clade composition should also be a natural phenomenon. A key factor for the establishment of the downstream gradient could be differences in stream characteristics and habitat preferences of the different Phoxinus clades. Correspondingly, differences in habitat preferences of Phoxinus from the Meuse (clade 10), characterised as rheophilic water column fish (Didier and Kestemont, 1996), and minnows from the Danube (clade 5b), described as stationary benthic (Balon et al., 1986), have been reported. Even at the smaller regional scale of North Rhine-Westphalia, differences in habitat preferences were previously described among streams in the distribution range of clade 5b, 10, and 11 (Biedermann and Finch, 2010). The distribution of Phoxinus in the Sieg basin could thus present a case of ecological species sorting, the prevailing perspective shaping aquatic metacommunities (Heino et al., 2015). In the metacommunity concept, the species sorting perspective emphasises that variation in community composition is driven by environmental variation among discrete habitats (Leibold et al., 2004; Leibold and Loeuille, 2015).
The failed establishment of allochthonous clade 5b Phoxinus in foothill streams suggest that the natural gradient in clade composition results from species sorting. Despite multiple stocking efforts with Phoxinus of clade 5b origin (Tab. 1, Appendix 2), the Agger and Naafbach in the foothill region were characterised by the absence of clade 5b haplotypes, while Phoxinus were generally missing in the Wahnbach (Fig. 2). Since Phoxinus undergo a longer phase of establishment, that can potentially last several years (Bless, 1992), their successful establishment could be extremely vulnerable to environmental influences like floods or pollution. Smaller fish, like Phoxinus, are often displaced during floods (Heggenes and Traaen, 1988; Thompson et al., 2011), while pollution was the main cause for the Sieg basin-wide extinctions of Phoxinus populations in the first place (Klinger, 2001; Landois et al., 1892; Wundsch, 1915). However, it seems rather unlikely that the stocking efforts failed due to environmental disturbances, as the external circumstances of the stocking efforts were generally favourable for Phoxinus establishment. Introduced Phoxinus populations in all three streams should have been relatively robust against displacement, as the streams were stocked from top to bottom, several times, and, in the case of the Agger, over four years. Extinction due to pollution seems unlikely, as pollution in the Sieg basin had generally declined (Arndt-Dietrich, 2002; Gellert, 2001b) and Phoxinus populations already existed in the Agger around the time of stocking (Borchard et al., 1986). Therefore, the failure of these stocking efforts is not linkable to environmental disturbances, but rather to the niche preferences of the introduced fish, which were not compatible with their new habitat.
Species sorting beyond the Sieg basin can further be recognised by the general distribution of clade 5b and 10 in the Rhine basin, which is in accordance with zoogeographic regions of Europe. While Phoxinus clade 10 is autochthonous to tributaries of the Rhine in the Atlantic region (Lower Rhine), Phoxinus clade 5b is autochthonous to Rhine tributaries of the Continental region (Middle Rhine and Upper Rhine). Streams in these regions differ in their general environmental characteristics (cf. Sundseth, 2009a, 2009b) and could thus provide different habitats, facilitating species sorting across the whole Rhine basin. Future research on the ecological niches of Phoxinus clade 5b and clade 10 is therefore necessary, to identify ecological differences that could promote species sorting.
4.5 Species determination in a cryptic species complex
With the occurrence of four distinct Phoxinus clades in the Sieg basin, three of which recognised as valid species (Palandačić et al., 2017), stakeholders expressed concerns about species identification in the field. Difficulties in distinguishing Phoxinus are commonly attributed to phenotypic variation within the different genetic clades (Bogutskaya et al., 2020; Collin and Fumagalli, 2015; Ramler et al., 2017). Successful species delimitation studies usually included external characteristics like scale counts, breast scale patch patterns, and morphometric measurements (Bayçelebi et al., 2024; Bogutskaya et al., 2023, 2020; Kottelat and Freyhof, 2007). However, these methods require prolonged handling of the dead fish, often in a laboratory, and are thus not suitable as a laypeople approach. A promising approach could be the investigation of the overall body shape and positioning of key morphological features like fins using geometric morphometrics, which had recently been used to identify shape variation among distinct Phoxinus populations (Baranov, 2020; Collin and Fumagalli, 2015; Ramler et al., 2017; Scharnweber, 2020). In addition, the approach of species differentiation on the basis of nuptial colouration was successful in distinguishing French Phoxinus species (Denys et al., 2020). The quantification of differences in body shape and colouration could provide Sieg stakeholders with simple characteristics to distinguish between the recorded clades. Yet, both approaches have not been applied on the four clades present in the Sieg and therefore require future analysis.
4.6 Incomplete lineage sorting and hybridisation
Including this study, genetic analyses for species delimitation in Phoxinus were mainly based on mitochondrial genes (Bogutskaya et al., 2023; Palandačić et al., 2022, 2020, 2017). However, taxonomic inferences from single-gene approaches, such as the barcoding method, are limited by natural processes like hybridisation, introgression, and/or incomplete lineage sorting (Chase et al., 2005; Funk and Omland, 2003; Moritz and Cicero, 2004; Nabholz, 2024). In the case of incomplete lineage sorting, phylogenies based on a single gene deviate from that of the species tree (Degnan and Salter, 2005), due to the retention of an ancestral polymorphism in a lineage until the next speciation event (Maddison, 1997). Incomplete lineage sorting in Phoxinus has previously been hypothesised between clade 1b-c and 9 (Palandačić et al., 2017) and might be identifiable in Phoxinus clade 10 and 5b. In both this study (Fig. 3) and in previous investigations (Denys et al., 2020; Esposito et al., 2024), genetic divergence of at least six mutational steps at the mitochondrial level were identified between clade 10 Phoxinus from English Channel tributaries (Seine and Normandy basins) and tributaries of the Southern Bight (Rhine and Meuse; Fig. 3c). Yet both groups are still assigned to clade 10 (Denys et al., 2020; Palandačić et al., 2022). Similarly, incomplete lineage sorting could exist between Phoxinus clade 5b from the Rhine and Danube basin. Phoxinus from the two basins are currently classified as the same clade, even though sharing of haplotypes between the two basins is rare (Fig. 3d; Esposito et al., 2024; Suh et al., 2023). Importantly, it must be noted that an analysis at the mitochondrial level is not sufficient to resolve incomplete lineage sorting. For this, an examination at the nuclear level, preferably of the whole genome is necessary for both clades.
Of the clades recorded in the Sieg, clades 5b and 10 have already been proven to hybridise (Palandačić et al., 2017). Since both clades are autochthonous in the Sieg basin, a natural contact zone with hybrid formation seems likely. Hybrid zones are confined regions in which genetically distinct populations meet, reproduce, and bear hybrids (Barton and Hewitt, 1985; Hewitt, 1988). The stocking efforts with locally allochthonous Phoxinus might have led to the establishment of at least two smaller and a larger, wide-ranging anthropogenic hybrid zone in the Sieg basin. Hybrid persistence, reproduction, and backcrossing in hybrid zones can lead to the establishment of a hybrid swarm (Harrison, 1993). Range expansions and invasive potential of hybrid swarms have previously been described in fish (Bourret et al., 2022; Glotzbecker et al., 2016; Rosenfield et al., 2004), crayfish (Perry et al., 2002) and plants (Ayres et al., 2008; Castillo et al., 2010). Accordingly, observed range expansions and growth of Phoxinus populations (Biedermann and Finch, 2010) could indicate that this is also the case in the Sieg basin. The Sieg basin is therefore an excellent system for studying ongoing hybridisation processes. However, Phoxinus in the Rhenish Massif were always characterised as ubiquitous, occurring in mass populations, and only began to decline as their habitats became increasingly polluted (Cornelius, 1865; Landois et al., 1892; v. d. Borne, 1881; Wundsch, 1915). The positive population development of Phoxinus in the Sieg basin should thus be seen as a return to a more natural fish community, which is locally set on the foundation of hybrid swarms.
The above-discussed limitations of the single-gene approach could be overcome through the combination of mitochondrial and nuclear markers (Monaghan et al., 2005; Sonnenberg et al., 2007; Vuataz et al., 2011). While phylogenetic inferences from nuclear genes in Phoxinus are often inconclusive or insufficient (Behrens-Chapuis et al., 2021; Palandačić et al., 2022, 2020, 2015), they have been shown to be helpful in the identification of Phoxinus hybridisations when combined with mitochondrial markers (Corral-Lou et al., 2019; Palandačić et al., 2022, 2017, 2015; Vucić et al., 2018). The next step to resolve the cryptic species complex conundrum in Phoxinus will be the application of reduced representation sequencing approaches or even whole genome re-sequencing. This is now possible with the recently published reference genome for P. phoxinus (Oriowo et al., 2025). Whole genome re-sequencing has been proven a successful method in studying hybridisation (Malde et al., 2017; v. Holdt et al., 2016) as well as species delimitation (Gu et al., 2023; Herrera et al., 2022; Hu et al., 2020; Petzold and Hassanin, 2020) and should thus be a suitable approach.
In conclusion, the present results challenge and revise the assumed natural ranges and autochthony of Phoxinus spp. in the Rhine basin. In the Ahr, clade 5b is the prevailing and exclusively autochthonous clade, while clade 10, historically identified as autochthonous, is identified as allochthonous. Conversely, in the Sieg basin, both clades 5b and 10 are autochthonous, but their natural ranges are distinct: clade 5b is primarily distributed in the mountainous regions, while clade 10 is prevalent in the foothill regions of the basin. A historical overlap in their natural distribution ranges led to the formation of a contact zone between these clades in the Sieg basin, overshadowed by anthropogenic introductions from other areas of clade 5b and 10 occurrences in the Rhine. The minor presence of additional allochthonous clades, namely clades 11 and 12 in the Sieg, and clade 10 in the Ahr basin, can be attributed to unintentional human-mediated introductions. This study also provides the first comprehensive evidence of a downstream gradient in Phoxinus clade composition and occurrence on a basin-wide scale. These patterns could be influenced by ecological niche preferences of the respective clades, supporting the concept of species sorting, which might play a significant role in shaping the distribution of Phoxinus.
Acknowledgments
The extent of field work conducted in this study would not have been possible without many helping hands, therefore the authors would like to thank the large number of field helpers involved in this study. Special thanks go to Fabian Herder and Gaël Denys, who contributed to the success of the fieldwork with experience and support. We further acknowledge Claudia Etzbauer and Jonas Astrin for their support with laboratory procedures. The conclusions drawn in this study would not have been possible without the wide-ranging background knowledge about the water bodies investigated. We would therefore like to thank the stakeholders involved with the surveyed waters. In particular the many fishing rights holders who gave their consent to the samplings and were available afterwards to answer questions about the stocking histories of their waters. We would also like to thank all those who provided samples from potential stocking source populations from all over Germany, and Zuzana Musilová for samples from the Elbe. Lastly, we would like to thank Anja Palandačić and Nina Bogutskaya for the fruitful scientific exchange and the sharing of experience and knowledge in working with Phoxinus.
Author contribution statement
MS and NS applied for permits. NS, TOO & MS collected specimens. NS performed stakeholder interviews and drafted the manuscript. LP and AB performed extractions and COI barcoding on Oxford nanopore. TOO extracted barcodes from Illumina reads. MS devised the project, performed DNA extraction for Illumina sequencing, and analysed all barcodes. All authors contributed to revising the manuscript.
Funding
This project granted to MS was funded by the Leibniz association under SAW-J96/2020.
Supplementary Material
Figure A1. Division of the Sieg basin into relevant sections, as identified by the stakeholder survey. Numbered sections indicated by small interruptions refer to river sections and fishing rights leases in accordance with Supplement 2. The inset with the map of Germany indicates the location of the study area.
Appendix 1. COI-Barcodes used in this study.
Appendix 2. Tabular breakdown of historical stocking events per stream and year.
Appendix 3. Extended results - detailed stocking history of the Sieg basin.
Access hereReferences
- Arndt-Dietrich I. 2002. Gewässergütebericht 2001 Nordrhein-Westfalen − Berichtszeitraum 1995-2000, Landesumweltamt Nordrhein-Westfalen, Essen, 273 p. [Google Scholar]
- Artaev ON, Bolotovskiy AA, Turbanov IS, Gandlin AA, Kutuzov AV, Levina MA, Melentev DA, Pozdeev IV, Borisov MY, Levin BA. 2024a. Forgotten for two centuries: redescription of Phoxinus isetensis (Georgi, 1775) (Cypriniformes, Leuciscidae) − the most widespread minnow in Europe. Zoosyst Evol 100: 1155–1173. [CrossRef] [Google Scholar]
- Artaev ON, Turbanov IS, Bolotovskiy AA, Gandlin AA, Levin BA. 2024b. Taxonomic revision of Phoxinus minnows (Leuciscidae) from Caucasus, with description of a new narrow-ranged endemic species. Zoosyst Evol 100: 291–308. [CrossRef] [Google Scholar]
- Astrin JJ, Stüben PE. 2008. Phylogeny in cryptic weevils: molecules, morphology and new genera of western Palaearctic Cryptorhynchinae (Coleoptera: Curculionidae). Invertebr Syst 22: 503–522. [CrossRef] [Google Scholar]
- Ayres DR, Zaremba K, Sloop CM, Strong DR. 2008. Sexual reproduction of cordgrass hybrids (Spartina foliosa x alterniflora) invading tidal marshes in San Francisco Bay. Divers Distrib 14: 187–195. [CrossRef] [Google Scholar]
- Balon EK, Crawford SS, Lelek A. 1986. Fish communities of the upper Danube River (Germany, Austria) prior to the new Rhein-Main-Donau connection. Environ Biol Fishes 15, 243–271. [CrossRef] [Google Scholar]
- Bandelt H, Forster P, Röhl A. 1999. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16: 37–48. [CrossRef] [PubMed] [Google Scholar]
- Baranov VY. 2020. Body Shape Variability of the Minnow Phoxinus phoxinus (Linnaeus, 1758) (Cyprinidae, Actinopterygii) in Large and Small Watercourses of the Sylva River Basin (Middle Urals). Biol Bull Russ Acad Sci 47: 1285–1292. [CrossRef] [Google Scholar]
- Barton NH, Hewitt GM. 1985. Analysis of Hybrid Zones. Annu Rev Ecol Evol Syst 16: 113–148. [CrossRef] [Google Scholar]
- Bayçelebi E, Aksu İ, Turan D. 2024. Description of a new species of Phoxinus from the Ergene River (Aegean Sea Basin) in Türkiye (Actinopterygii, Leuciscidae). Zoosyst Evol 100: 101–110. [CrossRef] [Google Scholar]
- Behrens-Chapuis S, Herder F, Geiger MF. 2021. Adding DNA barcoding to stream monitoring protocols − What's the additional value and congruence between morphological and molecular identification approaches? PLOS ONE 16: e0244598. [CrossRef] [PubMed] [Google Scholar]
- Benson AJ. 1999. Documenting over a century of aquatic introductions in the United States., In Claudi R, Leach JH eds. Nonindigenous Freshwater Organisms: Vectors, Biology, and Impacts. Boca Raton, FL: CRC Press LLC, pp. 1–31. [Google Scholar]
- Bennett KD. 1997. Evolution and Ecology: The Pace of Life. Cambridge University Press. [Google Scholar]
- Berg LS. 1948. Ryby presnykh vod SSSR i sopredel'nykh stran [freshwater fishes of the U.S.S.R. and adjacent countries], Vol. 1, Izdatelstvo Akademii Nauk SSSR, Moscow & Leningrad, 466 p. [Google Scholar]
- Bernery C, Bellard C, Courchamp F, Brosse S, Gozlan RE, Jarić I, Teletchea F, Leroy B. 2022. Freshwater fish invasions: a comprehensive review. Annu Rev Ecol Evol Syst 53: 427–456. [CrossRef] [Google Scholar]
- Bianco PG. 2014. An update on the status of native and exotic freshwater fishes of Italy. J Appl Ichthyol 30: 62–77. [Google Scholar]
- Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, Winker K, Ingram KK, Das I. 2007. Cryptic species as a window on diversity and conservation. Trends Ecol Evol 22: 148–155. [CrossRef] [PubMed] [Google Scholar]
- Biedermann R, Finch OD. 2010. Analyse der räumlichen und zeitlichen Verbreitung der Elritze in Nordrhein-Westfalen, Aqua Ecology, Oldenburg, 102 p. [Google Scholar]
- Birkmann J, Schüttrumpf H, Handmer J, Thieken A, Kuhlicke C, Truedinger A, Sauter H, Klopries EM, Greiving S, Jamshed A, Merz B, Solecki W, Kirschbauer L. 2023. Strengthening resilience in reconstruction after extreme events − Insights from flood affected communities in Germany. Int J Disaster Risk Reduct 96: 103965. [CrossRef] [Google Scholar]
- Blanck A, Tedesco PA, Lamouroux N. 2007. Relationships between life-history strategies of European freshwater fish species and their habitat preferences. Freshw Biol 52: 843–859. [CrossRef] [Google Scholar]
- Bless R. 1992. Einsichten in die Ökologie der Elritze Phoxinus phoxinus (L.) − praktische Grundlagen zum Schutz einer gefährdeten Fischart, Forschungsanstalt für Naturschutz und Landschaftsökologie, Bonn, 57 p. [Google Scholar]
- Bogutskaya NG, Naseka AM. 2004. Catalogue of agnathans and fishes of fresh and brackish waters of Russia with comments on nomenclature and taxonomy. KMK Scientific Press Ltd., Moscow, 389 p. [Google Scholar]
- Bogutskaya NG, Jelić D, Vucić M, Jelić M, Diripasko OA, Stefanov T, Klobučar G. 2020. Description of a new species of Phoxinus from the upper Krka River (Adriatic Basin) in Croatia (Actinopterygii: Leuciscidae), first discovered as a molecular clade. J Fish Biol 96: 378–393. [CrossRef] [PubMed] [Google Scholar]
- Bogutskaya NG, Diripasko OA, Palandačić A. 2023. Novel data support validity of Phoxinus chrysoprasius (Pallas, 1814) (Actinopterygii, Leuciscidae). Eur J Taxon 861: 1–20. [CrossRef] [Google Scholar]
- Borchard B, Brenner T, Steinberg L. 1986. Fische in Nordrhein-Westfalen, Ministerium für Umwelt, Raumordnung und Landwirtschaft des Landes Nordrhein-Westfalen, Düsseldorf, 127 p. [Google Scholar]
- Borgstrøm R. 1973. Spredning av ørekyte. Jakt-Fiske-Friluftsliv 102, 28–29. [Google Scholar]
- Bourret SL, Kovach RP, Cline TJ, Strait JT, Muhlfeld CC. 2022. High dispersal rates in hybrids drive expansion of maladaptive hybridization. Proc R Soc B Biol Sci 289: 20221813. [CrossRef] [PubMed] [Google Scholar]
- Brasseur MV, Astrin JJ, Geiger MF, Mayer C. 2023. MitoGeneExtractor: Efficient extraction of mitochondrial genes from next-generation sequencing libraries. Methods Ecol Evol 14: 1017–1024. [CrossRef] [Google Scholar]
- Britton JR. 2023. Contemporary perspectives on the ecological impacts of invasive freshwater fishes. J Fish Biol 103: 752–764. [CrossRef] [PubMed] [Google Scholar]
- Buj I, Marčić Z, Ćaleta M, Šanda R, Geiger MF, Freyhof J, Machordom A, Vukić J. 2017. Ancient connections among the European rivers and watersheds revealed from the evolutionary history of the genus Telestes (Actinopterygii; Cypriniformes). PLoS ONE 12: e0187366. [Google Scholar]
- Byers JE, Wright JT, Gribben PE. 2010. Variable direct and indirect effects of a habitat-modifying invasive species on mortality of native fauna. Ecology 91: 1787–1798. [CrossRef] [PubMed] [Google Scholar]
- Cambray JA. 2003. Impact on indigenous species biodiversity caused by the globalisation of alien recreational freshwater fisheries. Hydrobiologia 500: 217–230. [CrossRef] [Google Scholar]
- Canestrini G. 1864. Sul Phoxinus laevis Ag. In: Canestrini G, ed. Archivio per La Zoologia, l'anatomia e La Fisiologia, Museo di Storia naturale della R. Università di Modena, Modena 307–308. [Google Scholar]
- Carlton JT. 1996. Biological Invasions and Cryptogenic Species. Ecology 77: 1653–1655. [CrossRef] [Google Scholar]
- Casimiro ACR, Garcia DAZ, Vidotto-Magnoni AP, Britton JR, Agostinho AA, Almeida FSD, Orsi ML. 2018. Escapes of non-native fish from flooded aquaculture facilities: the case of Paranapanema River, southern Brazil. Zoologia 35: 1–6. [CrossRef] [Google Scholar]
- Castillo JM, Ayres DR, Leira-Doce P, Bailey J, Blum M, Strong DR, Luque T, Figueroa E. 2010. The production of hybrids with high ecological amplitude between exotic Spartina densiflora and native S. maritima in the Iberian Peninsula. Divers Distrib 16: 547–558. [CrossRef] [Google Scholar]
- Castorani MCN, Hovel KA. 2015. Invasive prey indirectly increase predation on their native competitors. Ecology 96: 1911–1922. [CrossRef] [PubMed] [Google Scholar]
- Chan FT, Beatty SJ, Gilles Jr AS, Hill JE, Kozic S, Luo D, Morgan DL, Pavia Jr RTB, Therriault TW, Verreycken H, Vilizzi L, Wei H, Yeo DCJ, Yiwen Z, Zięba G, Copp GH. 2019. Leaving the fishbowl: the ornamental trade as a global vector for freshwater fish invasions. Aquat Ecosyst Health Manag 22: 417–439. [CrossRef] [Google Scholar]
- Chase MW, Salamin N, Wilkinson M, Dunwell JM, Kesanakurthi RP, Haidar N, Savolainen V. 2005. Land plants and DNA barcodes: short-term and long-term goals. Philos Trans R Soc B Biol Sci 360: 1889–1895. [CrossRef] [PubMed] [Google Scholar]
- Chen X. 1988. A new species of Phoxinus from China (Pisces, Cypriniformes). Sinozoologia 6: 35–38. [Google Scholar]
- Clavero M, García-Berthou E. 2005. Invasive species are a leading cause of animal extinctions. Trends Ecol Evol 20: 110. [CrossRef] [PubMed] [Google Scholar]
- Collado GA. 2017. Unraveling cryptic invasion of a freshwater snail in Chile based on molecular and morphological data. Biodivers Conserv 26: 567–578. [CrossRef] [Google Scholar]
- Collin H, Fumagalli L. 2015. The role of geography and ecology in shaping repeated patterns of morphological and genetic differentiation between European minnows (Phoxinus phoxinus) from the Pyrenees and the Alps. Biol J Linn Soc 116: 691–703. [CrossRef] [Google Scholar]
- Cornelius C. 1865. Zug- und Wander-Fische In: Cornelius C, ed. Die Zug- und Wanderthiere aller Thierklassen, Julius Springer, Berlin, 151–209. [Google Scholar]
- Corral-Lou A, Perea S, Aparicio E, Doadrio I. 2019. Phylogeography and species delineation of the genus Phoxinus Rafinesque, 1820 (Actinopterygii: Leuciscidae) in the Iberian Peninsula. J Zool Syst Evol Res 57: 926–941. [CrossRef] [Google Scholar]
- Degnan JH, Salter LA. 2005. Gene tree distributions under the coalescent process. Evolution 59: 24–37. [Google Scholar]
- De Santis V, Delmastro GB, Vanetti I, Britton JR, Zaccara S. 2021. Species composition of introduced and natural minnow populations of the Phoxinus cryptic complex in the westernmost part of the Po River Basin (north Italy). Biol Invasions 23: 657–668. [CrossRef] [Google Scholar]
- Denys GPJ, Manne S. 2019. First record of Phoxinus csikii Hankó, 1922 (Actinopterygii, Cypriniformes) in France. Cybium 43: 199–202. [Google Scholar]
- Denys GPJ, Dettai A, Persat H, Daszkiewicz P, Hautecœur M, Keith P. 2020. Revision of Phoxinus in France with the description of two new species (Teleostei, Leuciscidae). Cybium 44: 205–237. [Google Scholar]
- Didier J, Kestemont P. 1996. Relationships between mesohabitats, ichthyological communities and IBI metrics adapted to a European river basin (The Meuse, Belgium). Hydrobiologia 341: 133–144. [CrossRef] [Google Scholar]
- Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR. 2016. Invasive predators and global biodiversity loss. Proc Natl Acad Sci 113: 11261–11265. [CrossRef] [PubMed] [Google Scholar]
- Dyldin YV, Orlov AM. 2016. Ichthyofauna of fresh and brackish waters of Sakhalin Island: An annotated list with taxonomic comments: 2. Cyprinidae-Salmonidae families. J Ichthyol 56: 656–693. [CrossRef] [Google Scholar]
- Dyldin YV, Orlov AM, Hanel L, Romanov VI, Fricke R, Vasil'eva ED. 2023. Ichthyofauna of the Fresh and Brackish Waters of Russia and Adjacent Areas: Annotated List with Taxonomic Comments. 2. Order Cypriniformes, Suborders Catostomoidei, Cobitoidei and Cyprinoidei. J Ichthyol 63: 636–686. [CrossRef] [Google Scholar]
- Esposito A, Denys GPJ, Haÿ V, Godeaux Q, Foata J, Quilichini Y. 2024. Multiple introduction pathways of non-native Phoxinus minnows (Teleostei: Leuciscidae) in Corsica revealed by its hidden diversity and their parasites. Biol Invasions 26: 2453–2474. [CrossRef] [Google Scholar]
- Feulner PGD, Kirschbaum F, Schugardt C, Ketmaier V, Tiedemann R. 2006. Electrophysiological and molecular genetic evidence for sympatrically occuring cryptic species in African weakly electric fishes (Teleostei: Mormyridae: Campylomormyrus). Mol Phylogenet Evol 39: 198–208. [CrossRef] [PubMed] [Google Scholar]
- Freyhof J. 1998. Die Fische und Neunaugen der Sieg in den Grenzen von Nordrhein-Westfalen. Decheniana 151: 183–194. [CrossRef] [Google Scholar]
- Freyhof J, Bowler D, Broghammer T, Friedrichs-Manthey M, Heinze S, Wolter C. 2023. Rote Liste und Gesamtartenliste der sich im Süßwasser reproduzierenden Fische und Neunaugen (Pisces et Cyclostomata) Deutschlands, Bundesamt für Natur, Bonn, 63 p. [Google Scholar]
- Frost WE. 1943. The Natural History of the Minnow, Phoxinus phoxinus. J Anim Ecol 12: 139–162. [CrossRef] [Google Scholar]
- Funk DJ, Omland KE. 2003. Species-Level Paraphyly and Polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annu Rev Ecol Evol Syst 34: 397–423. [CrossRef] [Google Scholar]
- Fong JJ, Blom MPK, Aowphol A, McGuire JA, Sutcharit C, Soltis PS. 2023. Editorial: Recent advances in museomics: revolutionizing biodiversity research. Front Ecol Evol 11: 427–456. [Google Scholar]
- Garcia-Raventós A, Martins FMS., Teixeira A, Sousa R, Froufe E, Varandas S, Lopes-Lima M, Beja P, Filipe AF. 2020. Origin and history of Phoxinus (Cyprinidae) introductions in the Douro Basin (Iberian Peninsula): an update inferred from genetic data. Biol Invasions 22: 2409–2419. [CrossRef] [Google Scholar]
- Gellert G. 2001a. Die Gewässergüteentwicklung der unteren Sieg, In: Seuter S, ed. Gewässergütebericht 2000–30 Jahre Biologische Gewässerüberwachung in Nordrhein-Westfalen, Landesumweltamt Nordrhein-Westfalen, Essen, 84–86. [Google Scholar]
- Gellert G. 2001b. Gewässergüte und ökologischer Zustand der Agger. In: Seuter S, ed. Gewässergütebericht 2000–30 Jahre Biologische Gewässerüberwachung in Nordrhein-Westfalen, Landesumweltamt Nordrhein-Westfalen, Essen, 87–90. [Google Scholar]
- Glotzbecker GJ, Walters DM, Blum MJ. 2016. Rapid movement and instability of an invasive hybrid swarm. Evol Appl 9: 741–755. [CrossRef] [PubMed] [Google Scholar]
- Gozlan RE, Britton JR, Cowx I, Copp GH. 2010. Current knowledge on non-native freshwater fish introductions. J Fish Biol 76: 751–786. [Google Scholar]
- Gu TT, Wu H, Yang F, Gaubert P, Heighton SP, Fu Y, Liu K, Luo SJ, Zhang HR, Hu JY, Yu L. 2023. Genomic analysis reveals a cryptic pangolin species. Proc Natl Acad Sci 120: e2304096120. [CrossRef] [PubMed] [Google Scholar]
- Harrison RG. 1993. Hybrids and Hybrid Zones: Historical Perspective In: Harrison RG ed. Hybrid Zones and the Evolutionary Process. Oxford: Oxford University Press, 3–12. [CrossRef] [Google Scholar]
- Hahn EE, Alexander MR, Grealy A, Stiller J, Gardiner DM, Holleley CE. 2022. Unlocking inaccessible historical genomes preserved in formalin. Mol Ecol Resour 22: 2130–2147. [CrossRef] [PubMed] [Google Scholar]
- Hebert PDN, Cywinska A, Ball SL, deWaard JR. 2003. Biological identifications through DNA barcodes. Proc Biol Sci 270: 313–321. [CrossRef] [PubMed] [Google Scholar]
- Heckel J, Kner R. 1858. Die Süsswasserfische der Österreichischen Monarchie mit Rücksicht auf die angrenzenden Länder. Verlag Wilhelm Engelmann, Leipzig, 388 p. [Google Scholar]
- Heggenes J, Traaen T. 1988. Downstream migration and critical water velocities in stream channels for fry of four salmonid species. J Fish Biol 32: 717–727. [CrossRef] [Google Scholar]
- Heino J, Melo AS, Siqueira T, Soininen J, Valanko S, Bini LM. 2015. Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshw Biol 60: 845–869. [CrossRef] [Google Scholar]
- Hensel MJS, Silliman BR, van de Koppel J, Hensel E, Sharp SJ, Crotty SM, Byrnes JEK. 2021. A large invasive consumer reduces coastal ecosystem resilience by disabling positive species interactions. Nat Commun 12: 6290. [CrossRef] [PubMed] [Google Scholar]
- Herrera ND, Bell KC, Callahan CM, Nordquist E, Sarver BAJ, Sullivan J, Demboski JR, Good JM. 2022. Genomic resolution of cryptic species diversity in chipmunks. Evolution 76: 2004–2019. [CrossRef] [PubMed] [Google Scholar]
- Hewitt GM. 1988. Hybrid zones-natural laboratories for evolutionary studies. Trends Ecol Evol 3: 158–167. [CrossRef] [PubMed] [Google Scholar]
- Hewitt GM. 2004. Genetic consequences of climatic oscillations in the Quaternary. Philos Trans R Soc Lond B Biol Sci 359: 183–195. [CrossRef] [PubMed] [Google Scholar]
- Hewitt GM. 1999. Post-glacial re-colonization of European biota. Biol J Linn Soc 68: 87–112. [CrossRef] [Google Scholar]
- Hu Y, Thapa A, Fan H, Ma T, Wu Q, Ma S, Zhang D, Wang B, Li M, Yan L, Wei F. 2020. Genomic evidence for two phylogenetic species and long-term population bottlenecks in red pandas. Sci Adv 6: eaax 5751. [Google Scholar]
- Jansen W, Kappus B, Böhmer J, Beiter T. 1999. Fish communities and migrations in the vicinity of fishways in a regulated river (Enz, Baden-Württemberg, Germany). Limnologica 29: 425–435. [CrossRef] [Google Scholar]
- Jia Y, Kennard MJ, Liu Y, Sui X, Chen Y, Li K, Wang G, Chen Y. 2019. Understanding invasion success of Pseudorasbora parva in the Qinghai-Tibetan Plateau: Insights from life-history and environmental filters. Sci Total Environ 694: 133739. [CrossRef] [PubMed] [Google Scholar]
- Jones PE, Champneys T, Vevers J, Börger L, Svendsen JC, Consuegra S, Jones J, Garcia de Leaniz C. 2021. Selective effects of small barriers on river-resident fish. J Appl Ecol 58: 1487–1498. [CrossRef] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30: 772–780. [CrossRef] [PubMed] [Google Scholar]
- Kirschbaum CL. 1865. Die Reptilien und Fische des Herzogthums Nassau. Verzeichniß und Bestimmungstabelle, In: Kirschbaum, CL, ed. Jahrbücher Des Vereins Für Naturkunde Im Herzogthum Nassau, Julius Riedner Verlagshandlung, Wiesbaden, 77–122. [Google Scholar]
- Klinger H. 2001. Fische unserer Bäche und Flüsse − Aktuelle Verbreitung, Entwicklungstendenzen, Schutzkonzepte für Fischlebensräume in Nordrhein-Westfalen, Ministerium für Umwelt und Naturschutz, Landwirtschaft und Verbraucherschutz des Landes Nordrhein-Westfalen, Düsseldorf, 200 p. [Google Scholar]
- Knebelsberger T, Dunz AR, Neumann D, Geiger MF. 2015. Molecular diversity of Germany's freshwater fishes and lampreys assessed by DNA barcoding. Mol Ecol Resour 15: 562–572. [CrossRef] [PubMed] [Google Scholar]
- Kottelat M. 1997. European freshwater fishes. An heuristic checklist of the freshwater fishes in Europe (exclusive of former Ussr), with an introduction for non-systematists and comments on nomenclature and conservation. Biologia 51: 1–271. [Google Scholar]
- Kottelat M. 2007. Three new species of Phoxinus from Greece and southern France (Teleostei: Cyprinidae). Ichthyol Explor Freshw 18: 145–162. [Google Scholar]
- Kottelat M, Freyhof J. 2007. Handbook of European Freshwater Fishes, Kottelat, Cornol, and Freyhof, Berlin, 646 p. [Google Scholar]
- Kusznierz J, Tagayev D, Sienkiewicz T, Paśko Ł. 2023. Molecular and osteological verification of the taxonomic status of Phoxinus sedelnikowi (Berg, 1908) (Teleostei: Leuciscidae). Eur Zool J 90: 113–125. [CrossRef] [Google Scholar]
- Landois H, Rade E, Westhoff F. 1892. Westfalens Fische In: Landois H, ed. Westfalens Tierleben: Die Reptilien, Amphibien und Fische in Wort und Bild, Verlag Ferdinand Schöningh, Paderborn, 161–432. [Google Scholar]
- Leibold MA, Loeuille N. 2015. Species sorting and patch dynamics in harlequin metacommunities affect the relative importance of environment and space. Ecology 96: 3227–3233. [CrossRef] [PubMed] [Google Scholar]
- Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A. 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7: 601–613. [CrossRef] [Google Scholar]
- Leigh JW, Bryant D. 2015. PopART: Full-feature software for haplotype network construction. Methods Ecol Evol 6: 1110–1116. [Google Scholar]
- Linders TEW, Schaffner U, Eschen R, Abebe A, Choge SK, Nigatu L, Mbaabu PR, Shiferaw H, Allan E. 2019. Direct and indirect effects of invasive species: Biodiversity loss is a major mechanism by which an invasive tree affects ecosystem functioning. J Ecol 107: 2660–2672. [CrossRef] [Google Scholar]
- Linnaeus C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis, Editio decima, reformata [10th revised edition], Impensis Direct, Laurentii Salvii, Holmiae [= Stockholm], 824 p. [Google Scholar]
- Maceda-Veiga A, Escribano-Alacid J, de Sostoa A, García-Berthou E. 2013. The aquarium trade as a potential source of fish introductions in southwestern Europe. Biol Invasions 15: 2707–2716. [Google Scholar]
- Maddison WP. 1997. Gene trees in species trees. Syst Biol 46: 523–536. [CrossRef] [Google Scholar]
- Malde K, Seliussen BB, Quintela M, Dahle G, Besnier F, Skaug HJ, Øien N, Solvang HK, Haug T, Skern-Mauritzen R, Kanda N, Pastene LA, Jonassen I, Glover KA. 2017. Whole genome resequencing reveals diagnostic markers for investigating global migration and hybridization between minke whale species. BMC Genom 18: 76. [CrossRef] [Google Scholar]
- Martin AP, Bermingham E. 2000. Regional endemism and cryptic species revealed by molecular and morphological analysis of a widespread species of Neotropical catfish. Proc R Soc Lond B Biol Sci 267: 1135–1141. [CrossRef] [PubMed] [Google Scholar]
- McEachran MC, Hofelich Mohr A, Lindsay T, Fulton DC, Phelps NBD. 2022. Patterns of Live Baitfish Use and Release among Recreational Anglers in a Regulated Landscape. North Am J Fish Manag 42: 295–306. [CrossRef] [Google Scholar]
- Milardi M, Aschonitis V, Gavioli A, Lanzoni M, Fano EA, Castaldelli G. 2018. Run to the hills: exotic fish invasions and water quality degradation drive native fish to higher altitudes. Sci Total Environ 624: 1325–1335. [CrossRef] [PubMed] [Google Scholar]
- Miró A, Ventura M. 2015. Evidence of exotic trout mediated minnow invasion in Pyrenean high mountain lakes. Biol Invasions 17: 791–803. [CrossRef] [Google Scholar]
- Miró A, Sabás I, Ventura M. 2018. Large negative effect of non-native trout and minnows on Pyrenean lake amphibians. Biol Conserv 218: 144–153. [CrossRef] [Google Scholar]
- Mohr S, Ehret U, Kunz M, Ludwig P, Caldas-Alvarez A, Daniell JE, Ehmele F, Feldmann H, Franca MJ, Gattke C, Hundhausen M, Knippertz P, Küpfer K, Mühr B, Pinto JG, Quinting J, Schäfer AM, Scheibel M, Seidel F, Wisotzky C. 2023. A multi-disciplinary analysis of the exceptional flood event of July 2021 in central Europe − Part 1: Event description and analysis. Nat Hazards Earth Syst Sci 23: 525–551. [CrossRef] [Google Scholar]
- Monaghan MT, Balke M, Gregory TR, Vogler AP. 2005. DNA-based species delineation in tropical beetles using mitochondrial and nuclear markers. Philos Trans R Soc B Biol Sci 360: 1925–1933. [CrossRef] [PubMed] [Google Scholar]
- Morais P, Reichard M. 2018. Cryptic invasions: a review. Sci Total Environ 613-614: 1438–1448. [CrossRef] [PubMed] [Google Scholar]
- Moritz C, Cicero C. 2004. DNA Barcoding: Promise and Pitfalls. PLOS Biol 2: e354. [CrossRef] [PubMed] [Google Scholar]
- Museth J, Hesthagen T, Sandlund OT, Thorstad EB, Ugedal O. 2007. The history of the minnow Phoxinus phoxinus (L.) in Norway: from harmless species to pest. J Fish Biol 71: 184–195. [CrossRef] [Google Scholar]
- Museth J, Borgstrøm R, Brittain JE. 2010. Diet overlap between introduced European minnow (Phoxinus phoxinus) and young brown trout (Salmo trutta) in the lake, Øvre Heimdalsvatn: a result of abundant resources or forced niche overlap? Hydrobiologia 642: 93–100. [CrossRef] [Google Scholar]
- Nabholz B. 2024. Incomplete lineage sorting explains the low performance of DNA barcoding in a radiation of four species of Western European grasshoppers (Orthoptera: Acrididae: Chorthippus). Biol J Linn Soc 141: 33–50. [CrossRef] [Google Scholar]
- Nitsche H, Hein W. 1909. Die Süsswasserfische Deutschlands ihre Kennzeichen, Fortpflanzung, Verbreitung und wirtschaftliche Bedeutung, 4th ed, Verlag des Deutschen Fischereivereins, Berlin, 82 p. [Google Scholar]
- NZO [Gesellschaft für landschaftsökologische Planung, Berwertung und Dokumentation m.b.h.], IFÖ [Institut für angewandte Ökologie]. 2007. Historische Verbreitungskarten mit Erläuterungen, Erarbeitung von Instrumenten zur gewässerökologischen Beurteilung der Fischfauna, Ministerium für Umwelt und Naturschutz, Landwirtschaft und Verbraucherschutz des Landes NRW, Albaum, 89 p. [Google Scholar]
- Oberdorff T, Guilbert E, Lucchetta JC. 1993. Patterns of fish species richness in the Seine River basin, France. Hydrobiologia 259: 157–167. [CrossRef] [Google Scholar]
- Oriowo TO, Chrysostomakis I, Martin S, Kukowka S, Brown T, Winkler S, Myers EW, Böhne A, Stange M. 2025. A chromosome-level, haplotype-resolved genome assembly and annotation for the Eurasian minnow (Leuciscidae: Phoxinus phoxinus) provide evidence of haplotype diversity. GigaScience 14: giae116. [CrossRef] [PubMed] [Google Scholar]
- Palandačić A, Bravničar J, Zupančič P, Šanda R, Snoj A. 2015. Molecular data suggest a multispecies complex of Phoxinus (Cyprinidae) in the Western Balkan Peninsula. Mol Phylogenet Evol 92: 118–123. [Google Scholar]
- Palandačić A, Diripasko OA, Kirchner S, Stefanov T, Bogutskaya NG. 2024. An integrative approach highlights the discrepancy in the genetic, phenotypic, and presumptive taxonomic structure of (Actinopterygii, Leuciscidae, Phoxininae) in Bulgaria. J Fish Biol 105: 214–238. [CrossRef] [PubMed] [Google Scholar]
- Palandačić A, Kruckenhauser L, Ahnelt H, Mikschi E. 2020. European minnows through time: museum collections aid genetic assessment of species introductions in freshwater fishes (Cyprinidae: Phoxinus species complex). Heredity 124: 410–422. [Google Scholar]
- Palandačić A, Naseka A, Ramler D, Ahnelt H. 2017. Contrasting morphology with molecular data: an approach to revision of species complexes based on the example of European Phoxinus (Cyprinidae). BMC Evol Biol 17: 184. [CrossRef] [PubMed] [Google Scholar]
- Palandačić A, Witman K, Spikmans F. 2022. Molecular analysis reveals multiple native and alien Phoxinus species (Leusciscidae) in the Netherlands and Belgium. Biol Invasions 24: 2273–2283. [CrossRef] [Google Scholar]
- Perry WL, Lodge DM, Feder JL. 2002. Importance of hybridization between indigenous and nonindigenous freshwater species: an overlooked threat to North American biodiversity. Syst Biol 51: 255–275. [CrossRef] [PubMed] [Google Scholar]
- Petzold A, Hassanin A. 2020. A comparative approach for species delimitation based on multiple methods of multi-locus DNA sequence analysis: a case study of the genus Giraffa (Mammalia, Cetartiodactyla). PLoS One 15: e0217956. [CrossRef] [PubMed] [Google Scholar]
- Piria M, Simonović P, Kalogianni E, Vardakas L, Koutsikos N, Zanella D, Ristovska M, Apostolou A, Adrović A, Mrdak D, Tarkan AS, Milošević D, Zanella LN, Bakiu R, Ekmekçi FG, Povž M, Korro K, Nikolić V, Škrijelj R, Kostov V, Gregori A, Joy MK. 2018. Alien freshwater fish species in the Balkans − Vectors and pathways of introduction. Fish Fish 19: 138–169. [CrossRef] [Google Scholar]
- Popp H, Lehr O. 2008. Renaturierungsprojekte in Hessen am Beispiel der Wisper und der Nidda − Versuch einer Erfolgsbewertung. Schrreihe Dtsch Rat Landespfl 81: 93–95. [Google Scholar]
- Rahel FJ. 2004. Unauthorized fish introductions: fisheries management of the people, for the people, or by the people? Am Fish Soc Symp 44: 431–443. [Google Scholar]
- Raj S, Kumar AB, Tharian J, Raghavan R. 2021. Illegal and unmanaged aquaculture, unregulated fisheries and extreme climatic events combine to trigger invasions in a global biodiversity hotspot. Biol Invasions 23: 2373–2380. [CrossRef] [Google Scholar]
- Ramler D, Palandačić A, Delmastro GB, Wanzenböck J, Ahnelt H. 2017. Morphological divergence of lake and stream Phoxinus of Northern Italy and the Danube basin based on geometric morphometric analysis. Ecol Evol 7: 572–584. [CrossRef] [PubMed] [Google Scholar]
- Ratnasingham S, Hebert PDN. 2007. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol Ecol Notes 7: 355–364. [CrossRef] [PubMed] [Google Scholar]
- Reier S, Bogutskaya N, Palandačić A. 2022a. Comparative Phylogeography of Phoxinus, Delminichthys, Phoxinellus and Telestes in Dinaric Karst: Which Factors Have Influenced Their Current Distributions? Diversity 14: 526. [CrossRef] [Google Scholar]
- Reier S, Kruckenhauser L, Snoj A, Trontelj P, Palandačić A. 2022b. The minnow Phoxinus lumaireul (Leuciscidae) shifts the Adriatic-Black Sea basin divide in the north-western Dinaric Karst region. Ecohydrology 15: e2449. [CrossRef] [PubMed] [Google Scholar]
- Reifenrath J, Schmieds U. 2001. Erfolge der Gewässerüberwachung an der oberen Sieg. In: Arndt-Dietrich I, ed. Gewässergütebericht 2001 Nordrhein-Westfalen − Berichtszeitraum 1995-2000, Landesumweltamt Nordrhein-Westfalen, Essen 79–83. [Google Scholar]
- Rodríguez CF, Bécares E, Fernández-Aláez M. 2003. Shift from clear to turbid phase in Lake Chozas (NW Spain) due to the introduction of American red swamp crayfish (Procambarus clarkii). Hydrobiologia 506: 421–426. [CrossRef] [Google Scholar]
- Rosenfield JA, Nolasco S, Lindauer S, Sandoval C, Kodric-Brown A, 2004. The Role of Hybrid Vigor in the Replacement of Pecos Pupfish by Its Hybrids with Sheepshead Minnow. Conserv Biol 18: 1589–1598. [CrossRef] [Google Scholar]
- Sayers EW, Bolton EE, Brister JR, Canese K, Chan J, Comeau DC, Connor R, Funk K, Kelly C, Kim S, Madej T, Marchler-Bauer A, Lanczycki C, Lathrop S, Lu Z, Thibaud-Nissen F, Murphy T, Phan L, Skripchenko Y, Tse T, Wang J, Williams R, Trawick BW, Pruitt KD, Sherry ST. 2021. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 50: D20– D26. [Google Scholar]
- Scharnweber K. 2020. Morphological and trophic divergence of lake and stream minnows (Phoxinus phoxinus). Ecol Evol 10: 8358–8367. [CrossRef] [PubMed] [Google Scholar]
- Schwevers U. 1990. Kartierung der Fischfaune im Gewässersystem der Kinzig. Verh Ges Ökol 19: 670–680. [Google Scholar]
- Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, García-Berthou E, Pascal M, Pyšek P, Sousa R, Tabacchi E, Vilà M. 2013. Impacts of biological invasions: what's what and the way forward. Trends Ecol Evol 28: 58–66. [CrossRef] [PubMed] [Google Scholar]
- Sonnenberg R, Nolte AW, Tautz D. 2007. An evaluation of LSU rDNA D1-D2 sequences for their use in species identification. Front Zool 4: 6. [CrossRef] [PubMed] [Google Scholar]
- Srivathsan A, Lee L, Katoh K, Hartop E, Kutty SN, Wong J, Yeo D, Meier R. 2021. ONTbarcoder and MinION barcodes aid biodiversity discovery and identification by everyone, for everyone. BMC Biol 19: 217. [CrossRef] [PubMed] [Google Scholar]
- Srivathsan A, Feng V, Suárez D, Emerson B, Meier R. 2023. ONTbarcoder 2.0: rapid species discovery and identification with real-time barcoding facilitated by Oxford Nanopore R10. 4. Cladistics 40: 192–203. [Google Scholar]
- Steinberg L, NZO [Gesellschaft für landschaftsökologische Planung, Berwertung und Dokumentation m.b.h.]. 1992. Fische unserer Bäche und Flüsse − Verbreitung, Gefährdung und Schutz in Nordrhein-Westfalen, Ministerium für Umwelt, Raumordnung und Landwirtschaft des Landes Nordrhein-Westfalen, Düsseldorf, 121 p. [Google Scholar]
- Suh J, Albo-Timor A, Aparicio E, Buchaca T, Caner J, Osorio V, Rovira Q, Puig M, Rocaspana R, Sabás I, Tiberti R, Ventura M. 2023. Espècies de veró i variabilitat genètica al Parc Nacional i en altres àrees d'alta muntanya, Generalitat de Catalunya, Barcelona, 186 p. [Google Scholar]
- Sundseth K. 2009a. Natura 2000 in the Atlantic Region. Publications Office of the European Union, Luxembourg, 11p. [Google Scholar]
- Sundseth, K., 2009b. Natura 2000 in the Continental Region. Publications Office of the European Union, Luxembourg, 11 p. [Google Scholar]
- Svirgsden R, Rohtla M, Albert A, Taal I, Saks L, Verliin A, Vetemaa M. 2018. Do Eurasian minnows (Phoxinus phoxinus L.) inhabiting brackish water enter fresh water to reproduce: Evidence from a study on otolith microchemistry. Ecol Freshw Fish 27: 89–97. [CrossRef] [Google Scholar]
- Tack E. 1941. Die Elritze (Phoxinus laevis), eine monographische Bearbeitung. Arch Hydrobiol 37: 321–425. [Google Scholar]
- Tack E. 1972. Die Fische des südwestfälischen Berglandes mit Einschluß von Möhnetalsperre und Ruhr. Decheniana 125: 63–77. [Google Scholar]
- Thompson LC, Cocherell SA, Chun SN, Cech JJ, Klimley AP. 2011. Longitudinal movement of fish in response to a single-day flow pulse. Environ Biol Fishes 90: 253–261. [CrossRef] [Google Scholar]
- Tiberti R, Buchaca T, Cruset Tonietti E, Iacobelli L, Maini M, Ribelli F, Pou Rovira Q, Ventura M. 2022. Minnow introductions in mountain lakes result in lower salmonid densities. Biol Invasions 24: 2285–2289. [CrossRef] [Google Scholar]
- Troschel FH. 1851. Ueber die Rümpchen. Verh Natur Ver Preuss Rheinl Westfal 8: 563–567. [Google Scholar]
- Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. 2000. Preparation of PCR-Quality Mouse Genomic DNA with Hot Sodium Hydroxide and Tris (HotSHOT). BioTechniques 29: 52–54. [CrossRef] [PubMed] [Google Scholar]
- Turan D, Bayçelebi E, Özuluğ M, Gaygusuz Ö, Aksu İ. 2023. Phoxinus abanticus, a new species from the Lake Abant drainage in Türkiye (Teleostei: Leuciscidae). J Fish Biol 102: 1157–1167. [CrossRef] [PubMed] [Google Scholar]
- Borne M. v. d. 1881. Die Fischerei-Verhältnisse des Deutschen Reiches, Oesterreich-Ungarns, der Schweiz und Luxemburgs, W. Moeser, Berlin, 304 p. [Google Scholar]
- V. Holdt BM, Cahill JA, Fan Z, Gronau I, Robinson J, Pollinger JP, Shapiro B, Wall J, Wayne RK. 2016. Whole-genome sequence analysis shows that two endemic species of North American wolf are admixtures of the coyote and gray wolf. Sci Adv 2: e1501714. [CrossRef] [PubMed] [Google Scholar]
- von Siebold CTE. 1863. Die Süsswasserfische von Mitteleuropa, Engelmann, Leipzig, 430 p. [Google Scholar]
- Vuataz L, Sartori M, Wagner A, Monaghan MT. 2011. Toward a DNA Taxonomy of Alpine Rhithrogena (Ephemeroptera: Heptageniidae) Using a Mixed Yule-Coalescent Analysis of Mitochondrial and Nuclear DNA. PLoS One 6: e19728. [CrossRef] [PubMed] [Google Scholar]
- Vucić M, Jelić D, Žutinić P, Grandjean F, Jelić M. 2018. Distribution of Eurasian minnows (Phoxinus: Cypriniformes) in the Western Balkans. Knowl Manag Aquat Ecosyst 419: 11. [Google Scholar]
- Walter S. 2013. Morphologie und Haplotypenverteilung von Phoxinus phoxinus (Linneaus, 1758) (Pisces: Telostei: Cyprinidae) in der Sieg. Rheinische Friedrich-Wilhelms-Universität, Bonn, 63 p. [Google Scholar]
- Webb AC. 2007. Status of non-native freshwater fishes in tropical northern Queensland, including establishment success, rates of spread, range and introduction pathways. J Proc R Soc New South Wales 140: 63–78. [CrossRef] [Google Scholar]
- Westermann F, Fischer J, Ehlscheid T, Wanner S, Prawitt O, Loch P, Wendling K. 2011. Gewässerzustandsbericht 2010 − Ökologische Bilanz zur Biologie, Chemie und Biodiversität der Fließgewässer und Seen, Landesamt für Umwelt, Wasserwirtschaft, und Gewerbeaufsicht, Mainz and Ministrium für Umwelt, Landwirtschaft, Ernährung, Weinbau und Forsten, Mainz, 222 p. [Google Scholar]
- Wittmack L. 1875. Beiträge zur Fischereistatistik des Deutschen Reiches, Deutscher Fischerei Verein, Berlin, 251 p. [Google Scholar]
- Wundsch HH. 1915. Fischerei und Industrie im Gebiet der Sieg und ihrer wichtigsten Zuflüsse. Z Fisch 16: 151–386. [Google Scholar]
- Zhang CG, Zhao YH. et al. 2016. Species Diversity and Distribution of Inland Fishes in China, Science Press, Beijing, 296 p. [Google Scholar]
Cite this article as: Sternberg N, Bodenheim A, Oriowo TO, Podsiadlowski L, Stange M. 2025. Human impacts on the distribution and genetic diversity of Eurasian minnows (Phoxinus: Leuciscidae) in the Rhenish Massif. Knowl. Manag. Aquat. Ecosyst., 426. 20. https://doi.org/10.1051/kmae/2025015
All Tables
Summary of stocking practices conducted in the Sieg basin. For a detailed description of the stocking efforts, please see Appendices 2 and 3 in the Online Supplementary Material.
All Figures
 |
Fig. 1 Distribution and COI clade composition of Phoxinus populations in the Ahr basin based on specimens collected in the framework of this study. The red rectangle in the inset shows the location of the Ahr in Germany. Circles represent fished sections: black empty ones with no Phoxinus caught, green empty ones with Phoxinus caught but not barcoded, filled circles for sections with Phoxinus caught and successfully barcoded. The size of the circles is relative to the number of barcoded specimens, the colour denotes the COI-clade identity according to Palandačić et al. (2020). The sampling location of historical specimens caught in the Ahr, first presented in Knebelsberger et al. (2015), is indicated by a black arrow and the years in which they were sampled. |
| In the text | |
 |
Fig. 2 Distribution and COI clade composition of Phoxinus populations in the Sieg basin based on specimens collected in the framework of this study. The red rectangle in the inset shows the location of the Sieg in Germany. Circles represent fished sections: black empty ones with no Phoxinus caught, green empty ones with Phoxinus caught but not barcoded, filled circles for sections with Phoxinus caught and successfully barcoded. The size of the circles is relative to the number of barcoded specimens, the colour denotes the COI-clade identity according to Palandačić et al. (2020). Grey continuous lines indicate the designated Sieg basin zonation into mountainous and foothill region, based on major basin borders (confluences, weirs and artificial waterfalls), administrative districts and geographical regions. Dashed grey lines indicate the sub-region basin divide between Sieg and Agger. All streams discussed in the text are labelled in the figure. The neotype locality of P. phoxinus is indicated by a black target symbol. A single record of clade 11 is indicated by a black arrow. |
| In the text | |
 |
Fig. 3 Clustering of mitochondrial cytochrome oxidase I (COI) haplotypes from different European basins. a. Haplotype network based on 925 COI fragments from Phoxinus specimens from various European freshwater basins (colour-coded) of potential introductory resource populations. b. Overview of the European freshwater basins from which haplotypes were included in the haplotype network. The separate haplotype networks for c. clade 10, d. clade 5b, and e. clade 12 include a further breakdown of investigated basins. Numbers identify relevant haplotypes discussed in the results and discussion sections. Clades according to Denys et al. (2020) and Palandačić et al. (2020): clade 5b (P. csikii), clade 9 (P. marsilii), clade 10 (P. phoxinus), clade 12 (P. septimaniae), clade 13 (P. bigerri), clade 16 (P. fayollarum), clade 23 (P. dragarum), clade 11 (P. cf. morella). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


