| Issue |
Knowl. Manag. Aquat. Ecosyst.
Number 422, 2021
|
|
|---|---|---|
| Article Number | 21 | |
| Number of page(s) | 12 | |
| DOI | https://doi.org/10.1051/kmae/2021022 | |
| Published online | 28 May 2021 | |
Research Paper
Substrate type selection in diatom based lake water quality assessment
Sélection du type de substrat dans l'évaluation de la qualité de l'eau des lacs basée sur les diatomées
1
Department of Algology, Mycology and Lichenology, University of Belgrade, Faculty of Biology, Institute of Botany and Botanical Garden “Jevremovac”, Takovska 43, 11000 Belgrade, Serbia
2
University of Belgrade, Institute of Chemistry, Technology and Metallurgy, National Institute of the Republic of Serbia, Center of Ecology and Technoeconomics, Njegoševa 12, 11000 Belgrade, Serbia
* Corresponding author: itrbojevic@bio.bg.ac.rs
Received:
15
February
2021
Accepted:
30
April
2021
Various studies report contrasting results on the substrate-type effect on diatom community composition, but the particularly important question is whether or not it affects diatom-based assessments of water quality. We investigated whether the substrate type is a significant predictor of the diatom community composition and if it affects lake water quality assessment based on diatom indices. This study took place in Sava Lake (Serbia). We used glass, ceramic, willow and yew tree tiles as artificial substrates for periphyton development, and pebbles from the lake littoral as natural substrate. Results revealed differences in both the diatom community composition and diatom indices values related to the substrates. A distinction was recognized between natural, artificial wooden, and artificial inert substrates. However, the final lake quality assessment based on diatom indices was more or less similar in all substrate types in our study, and depended on value ranges associated with water quality classification and on diatom index choices. Artificial substrates in our study did show potential as an alternative for natural substrate, but further studies are required, particularly in various types of lentic ecosystems to confirm our findings and support artificial substrate employment in lake water quality assessment.
Résumé
Diverses études rapportent des résultats contrastés sur l'effet du type de substrat sur la composition de la communauté de diatomées, mais la question particulièrement importante est de savoir si cela affecte ou non les évaluations de la qualité de l'eau basées sur les diatomées. Nous avons cherché à savoir si le type de substrat est un prédicteur significatif de la composition de la communauté de diatomées et s'il affecte l'évaluation de la qualité de l'eau des lacs basée sur les indices de diatomées. Cette étude a eu lieu dans le lac Sava (Serbie). Nous avons utilisé des carreaux de verre, de céramique, de saule et d'if comme substrats artificiels pour le développement du périphyton, et des galets du littoral du lac comme substrat naturel. Les résultats ont révélé des différences dans la composition de la communauté de diatomées et dans les valeurs des indices de diatomées liées aux substrats. Une distinction a été reconnue entre les substrats naturels, artificiels en bois et artificiels inertes. Cependant, l'évaluation finale de la qualité du lac basée sur les indices de diatomées était plus ou moins similaire dans tous les types de substrats de notre étude, et dépendait des plages de valeurs associées à la classification de la qualité de l'eau et des choix d'indices de diatomées. Les substrats artificiels dans notre étude ont montré un potentiel comme alternative au substrat naturel, mais des études supplémentaires sont nécessaires, en particulier dans divers types d'écosystèmes lentiques pour confirmer nos résultats et soutenir l'utilisation de substrats artificiels dans l'évaluation de la qualité de l'eau des lacs.
Key words: Periphyton / diatom index / artificial substrate / natural substrate
Mots clés : Périphyton / indice diatomées / substrat artificiel / substrat naturel
© I.S. Trbojević et al., Published by EDP Sciences 2021
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License CC-BY-ND (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. If you remix, transform, or build upon the material, you may not distribute the modified material.
1 Introduction
The Water Framework Directive (WFD) promotes the concept of ecological status in water quality assessments, utilizing a holistic approach. Apart from physical, chemical, and hydromorphological quality elements, it combines multiple ecological groups in water bodies − as a biological component (EU, 2000). Biological components consistently reflect the ecological status of the water bodies, since the communities assessments integrate responses to environmental conditions over time periods such as weeks or even years (Richards et al., 2020). Periphyton is one of the most important groups of biological indicators in the surface water, and diatoms as the predominant component of periphyton have a long history in water quality monitoring and assessment (Fisher and Dunbar, 2007). Diatoms are considered to reflect the relatively recent water quality (few weeks period) due to their generally short life span (Stevenson et al., 2010), which makes them the perfect indicator in the monitoring of recent water quality variations − for example during the summer season in urban lakes, when the anthropogenic pressure is intensified.
Diatom indices have been developed to determine the environmental gradients, i.e. standardized value ranges of diatom indices point to the specific water quality class. These indices are mainly developed and tested to be used in rivers and streams, however, periphyton is a valuable biological indicator in shallow lakes as well (Bennion et al., 2010). In the 21st century, this possibility was recognized, and appropriate indices for lakes began to be developed in order to comply with the requirements of the WFD (Bennion et al., 2014). So far, the following diatom indices for lakes have been developed: Trophic Diatom Index for Lakes (TDIL) in Hungary (Stenger-Kovács et al., 2007), Lake Trophic Diatom Index (LTDI) in the UK (Kelly et al., 2007) and diatom index for lakes in Germany (DISeen) (Schaumburg et al., 2004, 2007). Still, the most often used diatom index in general (and for lake water quality assessment) is IPS − Indice de Polluo-Sensibilité Spécifique (Trábert et al., 2017), which is considered to be the most precise, as it takes into account approximately 2000 diatom species, the most among all diatom indices (Tan et al., 2017).
Although diatom-based indices are used extensively for both stream and lake water quality assessments, a standardized sampling substrate is still not established, and a wide span of both natural and artificial substrates are delegated as suitable for sampling diatoms (Richards et al., 2020 and references therein). Still, when artificial substrates are used, there is a recommendation to allow colonization for at least four weeks, so that the early colonizers influence on the final result is reduced (Kelly et al., 1998; Fisher and Dunbar, 2007; Richards et al., 2020). Artificial substrate advantages over natural substrates are reflected in uniform habitat conditions (by which microhabitat effects on the final water quality evaluation are excluded), exposition over known periods of time, positioning in the water column, and deployment in any site of interest (MacDonald et al., 2012). All these reasons justify intensifying studies to evaluate the reliability of these substrate usages in standard water quality monitoring procedures.
Diatom community composition, particularly the adaptability of some life forms to colonise certain microhabitats, is shown to be substrate-dependent by one group of studies, while the other group showed the opposite (Richards et al., 2020 and references there in). The same authors suggest that although the composition of taxa may vary between substrates, it might not affect diatom-based assessments of water quality based on diatom indices. Their study confirmed this hypothesis, considering water quality assessments of streams based on the Diatom Species Index for Australian Rivers (Richards et al., 2020).
Hypotheses tested in this study were (a) the substrate type is a significant predictor of the diatom community composition in lakes and (b) substrate choice affects final lake water quality assessment based on diatom indices. We expected to detect variations in the diatom community related to the substrate types, but did not expect those differences to significantly reflect on the water quality assessment based on diatom indices. Sava Lake was used as a model ecosystem in this study, and the results from 4 types of artificial substrates − glass, ceramic, willow and yew tiles and stone as a natural substrate were compared.
2 Materials and methods
2.1 Study site
The Sava Lake is a shallow urban reservoir (44°47′02.28″N, 20°23′25.64″E; 73 m a.s.l) formed in 1967 by embanking Sava river arm. Water volume of Sava Lake is about 4 000 000 m3, average depth is 4.5 m (maximum recorded 12 m), it is about 4.4 km long and about 250 m wide (Trbojević et al., 2017, 2019, Jovanović et al., 2017). Sava Lake is located in Belgrade, the capital city of Serbia. It serves in supplying drinking water (by means of bank filtration) to Belgrade citizens, and it is extensively used for recreational activities, especially during the summer months, when many cafes and restaurants host visitors near the lake shore (Trbojević et al., 2017, 2019). In Serbia, the Belgrade region, and Sava Lake, a moderate continental climate is predominant, but climate change effects in recent years are evident. Jovanović et al. (2017) reported phytoplankton of the Sava Lake to be affected by extreme meteorological events (frequent rainfalls and subsequent flooding event in Serbia during the survey period in 2014).
2.2 Environmental parameters
Water transparency, temperature, and dissolved oxygen/saturation were measured in situ at each sampling occasion using a Secchi disk and a YSI ProODO Optical Dissolved Oxygen Instrument. At the same occasions, samples of water for laboratory analyses were taken using a Ruttner bottle, just below the water surface (approximately 0.3 m), and processed at the Institute of Public Health of Serbia, where all analyses were performed using standard analytical methods (APHA, 1995). All measurements were done and all samples were taken in the central part of the lake, where artificial substrates were submerged.
2.3 Experimental design
Artificial substrates (glass, ceramic, willow and yew tree tiles, uniform dimensions, 2.6 × 7.6 cm) were submerged into the central part of Sava Lake (11th July 2014), at a depth of 50 cm, 80 cm and 140 cm from the water surface, using acrylic holders attached to a floating buoy as a carrier, anchored in the central part of Sava Lake (Fig. 1), and they were continuously incubated for the next two months (8 weeks). Acrylic holders placed all tiles vertically oriented in the water column. Samples were collected weekly, from 20th July − 9th September (in total 8 sampling weeks, during July, August and September). Simultaneously with artificial substrates, the epilithic community was sampled from natural stone substrates (3 to 5 pebbles, 3 to 5 cm in diameter were scraped and one composite sample obtained) collected in the Sava Lake littoral zone (approx. 0.5 m depth) (Fig. 1). Artificial substrates needed to be placed in the central part of the Sava Lake to prevent potential vandalism and artificial carrier disturbance. Since Sava Lake is a recreational and touristic center, the shore is completely adapted to the anthropogenic activities and more than 100 000 visitors per day utilize this resort during the summer season.
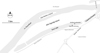 |
Fig. 1 Map showing sampling sites for artificial substrates (glass, ceramic, willow and yew tree tiles) and (b) natural substrate (pebble stone) in Sava Lake, Serbia. |
2.4 Diatom analyses
Tiles were transported and further processed in the Laboratory of Department of Algology, Mycology and Lichenology at the Institute of Botany and Botanical Garden “Jevremovac”, Faculty of Biology, University of Belgrade. Periphyton was scraped from tiles using a stainless steel razor blade tool. The collected material was acid treated (Taylor et al., 2005) and mounted on Naphrax® for diatom permanent slides preparation. Sampling design and sample processing are described in detail in Trbojević (2018) and Trbojević et al. (2017). Diatom taxonomic analyses and quantification (relative abundance) by counting at least 400 valves at each permanent slide were performed using a Carl Zeiss AxioImager. M1 microscope and a digital camera Axio Cam MRc 5 with Axio Vision 4.8 software. Taxonomic identification was done according to the standard literature (Hofmann et al., 2013, Lange-Bertalot et al., 2017 and others cited in Trbojević, 2018). The Shannon diversity index (H), equitability (Eh) and diatom indices were calculated using the OMNIDIA 6 software (Lecointe et al., 1993), and six diatom indices were considered for the water quality assessment of the Sava Lake water: Biological Diatom Index (IBD), Pollution Sensitivity Index (IPS), Trophic Diatom Index for Lakes (TDIL), Trophic Diatom Index (TDI), Trophic index (Rott TI), and Saprobic index (Rott SI). Water quality classes were determined according to Prygiel and Coste's water quality classification, based on the IBD index (Tab. 1) (Prygiel and Coste, 2000).
The Republic of Serbia legislation (Official Gazette of the RS 74/2011) recommends using only the IPS diatom index for assessing the ecological status of water body types that Sava Lake is assigned to. Peculiarly, according to the national water body classification (Official Gazette of the RS 96/2010) Sava Lake is classified as a natural lake, which is not in accordance with the way this urban reservoir was formed − by embanking the arm of the Sava river. Considering the same legislation, the ecological status of Sava Lake is to be determined by the IPS diatom index, with the following class ranges: I class >14, II class 10–14, III class 8–10, IV class 6–8 and V class <6 (Official Gazette of the RS 74/2011).
Colored sheme of water quality classification based on IBD index values (according to Prygiel and Coste, 2000).
2.5 Statistical analyses
Redundancy analyses were performed − two analyses focusing on diatom taxa, and two analyses focusing on diatom indices. Data relating to the period after 4 weeks of incubation of artificial substrates were considered for all analyses (from 10th August on), following the recommendation to allow colonization for at least four weeks, so that the early colonizers influence on the final result is reduced (Kelly et al., 1998; Fisher and Dunbar, 2007; Richards et al., 2020). Data from all artificial substrates and the natural one were used in the mentioned analyses. A separate redundancy analysis that was performed on data from artificial substrates in relation to incubation depths (50 cm, 80 cm and 140 cm) which were included as explanatory variables, showed that depth as a factor had no significance. Because of that, depth was not further subjected in analyses (though all the data were included).
Diatom taxa were first observed in relation to physical and chemical water parameters and sampling months. Explanatory variables − physical and chemical water parameters were tested for significance prior to analysis, and only those that were significant (P < 0.05) were included in the ordination diagram. Significant parameters were conductivity (Cond), pH and turbidity (Turb). Months of sampling (August and September) were included as supplementary variables. The twenty best fitted taxa were shown on the RDA ordination diagram. The linear method was chosen since the gradient for the first analysis was 2.4 SD units long. Additional RDA was done when substrate types were used as explanatory variables and observed in relation to diatom taxa, where as above, the twenty best fitted are shown on the ordination diagram.
Diatom indices were also observed in relation to explanatory and supplementary variables, in the same way as diatom taxa. The explanatory variables (physical and chemical water parameters) were preselected, and conductivity (Cond), permanganate index (a conventional measure of the contamination by organic and oxidizable inorganic matter in a water sample, PI), pH, water temperature (T) and turbidity (Turb) showed significance. The same supplementary variables were included as in the first mentioned RDA − months of sampling. The linear method was chosen since the gradient was 0.4 SD units long. As with diatom taxa, additional RDA was done when substrate types were used as explanatory variables.
CANOCO program for Windows, Version 5.0 (Ter Braak and Šmilauer, 2012) was used for all multivariate analyses.
3 Results
A detailed review of Sava Lake's environmental parameters dynamic during this study period has already been reported (Jovanović et al., 2017, Trbojević et al., 2017, 2019) and these parameters were used in multivariate analyses, in terms of explaining the variability of diatom community composition and diatom indices.
3.1 Diatom community composition and diversity (raw data)
A complete list of diatoms detected in this study consisted of 98 taxa when both artificial and natural substrates are considered, as is presented in Qualitative inventory (Appendix A). Taxa that occurred in quantitative analyses (65) are further marked with the codes retrived from Omnidia.
Quantitative analyses of diatom community composition in periphyton from the stone substrate showed that in all samples Achnanthidium minutissimum was dominant (in average 28%), while the subdominant Achnanthidium straubianum (in average 9%) and Navicula cryptotenelloides (in average 13%) alternated. Only on the second sampling date (27th July) Halamphora montana (14%) occurred in the subdominant position. The significant percentage in all samples was also distributed among the taxa Encyonopsis microcephala, Encyonopsis subminuta, Navicula antonii and Pantocsekiella ocellata. In periphyton from glass substrate, and the most samples from ceramic substrate, A. minutissimum was the dominant taxon (in average 50% on glass and 35% on ceramic), while on wooden substrates mostly E. microcephala (in average 18%) and occasionally E. subminuta and Cymbella affiniformis (in average 13% both) were dominant.
Diversity and equitability of the diatom community in all tested substrates are presented comparatively in Figure 2a and 2b. Both diversity index and equitability had high values in general (H > 3 on stone, and in almost all samples after 7–8 weeks of incubation), but still a bit lower in all artificial substrates in comparison to the natural substrate. The diversity index on stone varied between 2.8 and 3.9, while on artificial substrates it ranged from 0.9 to 3.4. Equitability on stone ranged from 0.74 to 0.83, and on artificial substrates from 0.3 to 0.9. When comparing artificial substrates among each other, diverisity and equitability were higher in wooden than in inert (glass and ceramic) substrates.
 |
Fig. 2 Dynamics of (a) diversity and (b) equitability of the diatom community during the study period in Sava Lake. |
3.2 Diatom community composition in relation to environmental parameters and different substrates
The relationship between physical and chemical water parameters that showed significance and the 20 best fitted diatom taxa is represented using RDA (F = 2.0, P = 0.03) (Fig. 3a). On this diagram, two major groups of taxa can be distinguished along the environmental gradient. The first group of taxa, the most represented in August, is placed on the right side of the ordination diagram and it is correlated positively with turbidity (Gomphonema tergestinum, Amphora pediculus, Fragilaria vaucheriae, Cocconeis placentula var. placentula, Melosira varians, Gomphonema varioreduncum, Encyonema auerswaldii). The second group in the left lower part of the ordination diagram is positively correlated with conductivity and pH and was representative for September (Ulnaria acus, Lindavia radiosa, Brachysira vitrea, Brachysira neoexilis, Gyrosigma kuetzingii, Geissleria decussis, Navicula radiosa, Staurosira brevistriata, A. minutissimum var. jackii, Placoneis clementioides, Caloneis cf. lancettula).
RDA demonstrating the relationship between diatom taxa and substrates used as explanatory variables is shown in Figure 3b. RDA was significant (F = 13.9, P = 0.002) and it described 48.88% variability in the data. Groups of taxa were clearly distinguishable, concentrated around the stone as natural substrate on one side, glass and ceramic as inert artificial substrates on the other, and wooden artificial substrates on the third side. The majority of taxa correlated with stone − markedly representatives of Navicula and Nitzschia, indicating that (particularly these taxa) the diversity of the diatom community was much better represented in natural substrate. A. minutissimum and A. minutissimum var. jackii were the most frequently encountered on glass and ceramic, while M. varians, C. affiniformis and B. vitrea seemed to prefer wooden substrates.
 |
Fig. 3 The relationship between diatom taxa and (a) environmental parameters and (b) substrates. |
3.3 Diatom indices and their relation to environmental parameters and different substrates
Diatom indices are presented in Figure 4, characterized by number values (0–20), and colored to indicate the water quality class they point to according to Table 1. Considering Prygiel and Coste's (2000) categorization, the water quality of Sava Lake was very good according to IBD, regardless of substrate type; IPS on glass substrate uniformly indicated very good quality, on ceramic mostly very good and on wooden substrates mostly good water quality. TDIL was overall uniform on all substrates − pointing to good water quality, except in very few cases in wooden and stone substrate when indicated moderate rank of water quality. TDI showed a similar trend, with two occasions when indicated water quality was bad (willow and yew) and very good (yew). Rott TI indicated prevalently moderate water quality on stone and wooden substrates, while on ceramic two times in a row bad quality was indicated (17th and 24th August). Good water quality indication prevailed on glass. According to Rott SI on all artificial substrates, water quality was mainly very good, and occasionally good, while on the stone it was uniformly good. When all indices are taken into account, it could be observed that artificial substrates in general indicated slightly better water quality in comparison to the natural substrate (stone). Especially inert substrates indicated better water quality − mainly very good and good. Wooden substrates gave more similar results to the natural substrate ranking water quality as mainly good, but often also moderate. When indication of water quality (if Prigiel and Coste's scale for water quality classification is considered) across the substrates are reviewed, it could be seen that only IBD and TDIL gave uniform results regardless of the substrate type.
When national legislative is considered, according to IPS values obtained upon the diatom community on the natural substrate, the ecological status of Sava Lake belonged to the first class, except on 17 August and 9 September, when values were very slightly below the first class boundary (13.9 and 13.8, respectively), placing Sava Lake in the second class of ecological status (Official Gazette of the RS 74/11). IPS values from all artificial substrates unequivocally placed Sava Lake in the ecological status of first class (disregarding the value of 13.6 obtained only once on willow substrate on 24th August).
When considering indices values obtained in the first 4 weeks of artificial substrates incubations (namely after 2 and 3 weeks of incubation, since the abundance of diatoms after only one week of incubation was insuffisicient for diatom indices calculation) and after the initial period of 4 weeks, it's obvious that not much variation in obtained values could be noticed (Fig. 4). Obtained values for diatom indices were in both periods similar, indicating that those could be considered representative even after only 2 weeks of incubation.
The relationship between physical and chemical water parameters that showed significance and diatom indices is represented using RDA (F = 3.5, P = 0.006) (Fig. 5a). It can be noticed that all index vectors are oriented toward the left side of the RDA ordination diagram. All of them positively correlated with conductivity and pH, and negatively with the other three explanatory variables − water temperature, permanganate index and turbidity. The values of all indices, except TDIL, were the highest in September, while the values of TDIL were similar in August and September (but slightly higher in September).
The second diagram shows the relationship between the indices and substrates used as explanatory variables (Fig. 5b). A 15.05% variability in the data was described (F = 2.6, P = 0.032). According to this analysis, TDIL, TDI, Rott TI and IPS pointed to better water quality when obtained from inert substrates, while Rott SI had somewhat higher values on wooden substrates. Interestingly, RDA placed stone substrate in the upper part of the ordination diagram, indicating that the obtained values for diatom indices were in general lower in natural substrate in comparison to all the artificial ones, and most markedly when IBD was considered (and to some extent IPS and Rott SI). Still, when TDIL, TDI, Rott TI were considered, the results from stones were higher in comparison to the wooden ones (which poorly/negatively correlated with this group of indices).
 |
Fig. 4 Diatom indices characterized by number values (0–20), and also by water quality classes they indicate according Prygiel and Coste's (2000) categorization. Empty fields are for the lost/damaged samples. |
 |
Fig. 5 The relationship between diatom indices and (a) environmental parameters and (b) substrates. |
4 Discussion
Considering the first hypothesis defined in the aim of this study (the substrate type is a significant predictor of the diatom community composition in lakes), our analyses indicated that diatom community composition could clearly be distinguished between substrate type groups − natural, artificial wooden substrates (willow and yew) and artificial inert (glass and ceramic) substrates.
Even with the raw results of quantitative analyses of the diatom community composition, differentiation between substrates could be anticipated, as A. minitissimum was found to be dominant on stone and inert artificial substrates, while wooden substrates were characterized mainly by prevalence of Encyonopsis spp.. Sabater et al. (1998) noticed similar taxa preference toward substrate. In their study, adnate forms (Achnanthidium spp.) were more abundant on flat ceramic tiles in comparison to the wooden tiles. Surface microstructure, i.e. heterogeneity of wooden substrate was proposed as an explanation for prevalence of the other morphological forms − colonial, on polysaccharide stalkes, actively mobile (Cymbella spp., Diatoma spp. and Navicula spp.). Our results also pointed to the preference of the smooth inert substrates (stone, glass, ceramic) by A. minutissimum, but wooden substrates were preferred by Encyonopsis spp. Both Achnanthidium spp. and Encyonopsis spp. belong to the same groups according to the life form (Berthon et al., 2011) and ecological guild (Passy, 2007) classifications. Thus, theoretically smoothness/roughness of the substrate should not be in the base of these taxa different substrate preferences. Another plausible explanation for this could be the inorganic (stone, glass, ceramic) and organic (wood) origin of substrates. Still, high abundance and occasional dominance of C. affiniformis on the wooden substrate in our study does insinuate that heterogeneity in surface microstructure of the wooden tiles can be the cause of substrate preference by diatoms in general (Sabater et al., 1998). Concerning diatom diversity, another parallel of our results with Sabater et al. (1998) can be drawn. In our study, a higher diversity on the wooden substrate in comparison to the inorganic artificial substrates was noticed (Fig. 2), again insinuating the importance of substrate microhabitat in shaping diatom community. But, in general, diversity on artificial substrates was slightly lower in comparison to the natural substrate, even after 8 weeks of incubation (Fig. 2), possibly reflecting discrepancies in colonization time between these substrates (Richards et al., 2020).
Multivariate analyses confirmed that the diatom community structure is dependent on environmental parameters (Fig. 3a), again confirming the strong bioindicating capacity of these organisms. In our study, the strongest drivers in diatom community shaping were turbidity, pH and conductivity, which could be considered clear indicators of anthropogenic pressure on urban lakes during the summer period (Fidlerová and Hlúbiková, 2016). Another analyses pronounced strong substrate influence on diatom community structure, differentiating three groups of substrates − natural, inert artificial and wooden artificial (Fig. 3b). The highest diatom diversity was indisputably related to the natural − stone substrate, and diversity of motile guild representatives (Navicula spp. and Nitzschia spp.) indicated high nutrient biofilms, where these taxa have competitive advantages (Passy, 2007). Our analyses associated M. varians, B. vitrea and C. affiniformis with wooden substrates. Considering autecological features and guild classification of these taxa, they support heterogeneity in surface microstructure of the wooden substratates as distinguishing factors in comparison to the smooth inert substrates, which were clearly preferred by exclusively adnate, pioneer forms (Achnanthidium spp.) (Passy, 2007; Berthon et al., 2011; Rimet and Bouchez, 2012). Differences in diatom guild distribution in natural and artificial substrate (motile guild representatives associated with stone, while low and few high guild representatives associated with artificial substrates), except from the colonizatition time difference, could reflect different grazing pressure in littoral and central part of Sava Lake (where artificial substrates were deployed) (Berthon et al., 2011). A slightly different trophic environment (Rimet et al., 2016) could be the base of these differences, considering that the shore of Sava Lake is entirely adapted for recreation and tourism, which may influence water quality especially in the littoral zone, where the anthropogenic activities are the most intensive. However, Bere and Tundisi (2011) showed in their study that common diatom species had strong preferences for natural (especially macrophytes) substrate over artificial substrates, supporting substrate specificity of the diatom community composition stressed out by our results.
The answer to the second hypothesis (substrate choice affects final lake water quality assessment based on diatom indices) is complex. According to our results, final lake quality assessment based on diatom indices was more or less similar in all substrate types, but dependent on index choice and the selected span of boundaries on the scale for water quality classification (we used Prygiel and Coste's categorization and National legislative guidelines and both indicated good comparability between substrates), differences could be observed. IBD and TDIL gave mostly uniform results in terms of water quality class according to Prygiel and Coste's categorization, regardless of the substrate type, and so did IPS, when the ecological classes categorization according to Serbian national legislation was considered (but not also Prygiel and Coste's categorization). Nevertheless, when raw values of obtained indices were analyzed, our results pointed to the same grouping pattern as for the diatom communities (though less expressive) − natural, arftificial wooden (willow and yew) and artificial inert (glass and ceramic) substrates could be distinguished and the results from these groups of substrates differed.
Looking at the raw values of the diatom indices (Fig. 4), it is already noticeable that dependant on the chosen index and water classification scale, differences related to the substrates are present. Considering Prigiel and Coste's classification, glass and ceramic seem to point to overall better water quality in comparison to the wooden and natural substrates. This indicates the possibility of wooden substrates to serve as a nutrient source for the periphytic algae (Zhang et al., 2013), developing a diatom community which suggests higher nutrient states. Thus, inert substrates should be considered more suitable for use in terms of diatom based biomonitoring, in comparison to the substrates of organic origin, such as wood. Nevertheless, longer colonization time and anthropogenic pressure in the littoral could partially contribute to the lower values of diatom indices detected on stone substrate, which is a clear reflection of previously discussed differences in diatom guilds distribution in natural and artificial substrates. IBD and TDIL gave the most uniform results in terms of water quality class, regardless of the substrate type. TDIL is the only metric developed specifically for lakes, to assess the trophic status of Hungarian lakes (Stenger-Kovács et al., 2007), among others used in our study. Our results clearly indicate that this index has the potential for application in routine assessment of ecological status and potential of Serbian lakes and reservoirs. According to the values for IPS (which is the only diatom index that Serbian national legislation consider for Sava Lake), results were also uniform across the substrates, and Sava Lake water ecological status was in the range of the first class.
Multivariate analyses, considering diatom indices, also revealed the same grouping pattern as for the diatom communities (though less expressive), i.e. natural, arftificial wooden (willow and yew) and artificial inert (glass and ceramic) substrates could be distinguished and the results from these types of substrates differed (Fig. 5b). The values of diatom indices were in general lower in the natural substrate in comparison to the artificial ones, and most markedly when IBD was considered (and to some extent IPS). Our analyses also revealed that all surveyed diatom indices showed an ascending trend along the gradient of conductivity and pH value, while they negatively correlated with water temperature, permanganate index and turbidity (Fig. 5a), confirming that the chosen diatom metrics all are reliable indicators of an integrated effect of different pressures reflected by physical and chemical water parameters (Fidlerová and Hlúbiková, 2016).
In conclusion, our study revealed differences in both diatom community composition and diatom indices values related to the substrates periphyton was developed on. Still, the robustness of diatom indices could be noticed, and final lake quality assessment based on diatom indices was more or less similar in all substrate types, but dependent on the selected span of boundaries on the scale for water quality classification and on diatom index choice. Artificial substrates employed in Sava Lake did show potential as good alternatives for natural substrate, but further studies are required to confirm our results, particularly in various types of lentic ecosystems, to support artificial substrate employment in lake water quality assessment.
Acknowledgments
This work was financially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 451-03-9/2021-14/ 200178 and 451-03-9/2021-14/200026). Authors are thankful to Ms Nevena Trbojević and Ms Nadežda Buntić for language corrections of the manuscript.
Appendix
Qualitative inventory − complete list of diatoms detected, when both artificial and natural substrates are considered. For taxa that occurred in quantitative analyses, codes (from Omnidia) are listed.
Codes labeled with * are for the taxa used for IBD calculation.
References
- APHA. 1995. Standard Methods for the Examination of Water and Wastewater. Washington DC: American Public Health Association. [Google Scholar]
- Bennion H, Sayer CD, Tibby J, Carrick HJ. 2010. Diatoms as indicators of environmental change in shallow lakes. In Smol PJ, Stoermer FE, eds. The diatoms: applications for the environmental and earth sciences. Cambridge: Cambridge University Press, 152–168. [Google Scholar]
- Bennion H, Kelly MG, Juggins S, Yallop ML, Burgess A, Reddihough G, Jamieson J, Krokowski J. 2014. Assessment of ecological status in UK lakes using benthic diatoms. Freshw Sci 33: 639–654. [CrossRef] [Google Scholar]
- Bere T, Tundisi JG. 2011. The effects of substrate type on diatom-based multivariate water quality assessment in a tropical river (Monjolinho), São Carlos, SP, Brazil. Water Air Soil Pollut 216: 391–409. [Google Scholar]
- Berthon V, Bouchez A, Rimet F. 2011. Using diatom life-forms and ecological guilds to assess organic pollution and trophic level in rivers: a case study of rivers in south-eastern France. Hydrobiologia 673: 259–271. [Google Scholar]
- European Union. 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Official Journal of the European Communities L327: 1–73. [Google Scholar]
- Fidlerová D, Hlúbiková D. 2016. Relationships between benthic diatom assemblages' structure and selected environmental parameters in Slovak water reservoirs (Slovakia, Europe). Knowl Manag Aquat Ecosyst 417: 27. [Google Scholar]
- Fisher J, Dunbar MJ. 2007. Towards a representative periphytic diatom sample. Hydrol Earth Syst Sci 11: 399–407. [CrossRef] [Google Scholar]
- Hofmann G, Werum M, Lange-Bertalot H. 2013. Diatomeen im Süßwasser − Benthos von Mitteleuropa: Bestimmungsflora Kieselalgen für die ökologische Praxis, Koeltz Scientific Books, Königstein, p. 908. [Google Scholar]
- Jovanović J, Trbojević I, Subakov Simić G, Popović S, Predojević D, Blagojević A, Karadžić V. 2017. The effect of meteorological and chemical parameters on summer phytoplankton assemblages in an urban recreational lake. Knowl Manag Aquat Ecosyst 418: 48. [CrossRef] [Google Scholar]
- Kelly MG, Cazaubon A, Coring E, Dell'Uomo A, Ector L, Goldsmith B, Guasch H, Hürlimann J, Jarlman A, Kawecka B, Kwandrans J, Laugaste R, Lindstrøm EA, Leitao M, Marvan P, Padisák J, Pipp E, Prygiel J, Rott E, Sabater S, van Dam H, Vizinet J. 1998. Recommendations for the routine sampling of diatoms for water quality assessments in Europe. J Appl Phycol 10: 215–224. [Google Scholar]
- Kelly MG, Juggins S, Bennion H, Burgess A, Yallop M, Hirst H, King L, Jamieson J, Guthrie R, Rippey B. 2007. Use of diatoms for evaluating ecological status in UK freshwaters, Environment Agency Science Report No. SC030103, Environment Agency, Bristol. [Google Scholar]
- Lange-Bertalot H, Hofmann G, Werum M, Cantonati M. 2017. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment. English edition with updated taxonomy and added species, Koeltz Botanical Books, Schmitten-Oberreifenberg, pp. 942. [Google Scholar]
- Lecointe C, Coste M, Prygiel J. 1993. “Omnidia”: software for taxonomy, calculation of diatom indices and inventories management. Hydrobiologia 269–270: 509–513. [Google Scholar]
- MacDonald LA, Balasubramaniam AM, Hall RI, Wolfe BB. 2012. Developing biomonitoring protocols for shallow Arctic lakes using diatoms and artificial substrate samplers. Hydrobiologia 683: 231–248. [Google Scholar]
- Official Gazette of the RS 96/2010: Regulation on establishment of surface and groundwater bodies. [Google Scholar]
- Official Gazette of the RS 74/2011: Regulation on parameters of ecological and chemical status of surface waters, and parameters of chemical and quantitative status of ground waters. [Google Scholar]
- Passy SI. 2007. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquat Bot 86: 171–178. [Google Scholar]
- Prygiel J, Coste M. 2000. Guide méthodologique pour la mise en œuvre de l'Indice Biologique Diatomées NF T 90-354, Agence de l'eau Artois Picardie, Douai, p. 340. [Google Scholar]
- Richards J, Tibby J, Barr C, Goonan P. 2020. Effect of substrate type on diatom-based water quality assessments in the Mount Lofty Ranges, South Australia. Hydrobiologia 847: 3077–3090. [Google Scholar]
- Rimet F, Bouchez A. 2012. Life-forms, cell-sizes and ecological guilds of diatoms in European rivers. Knowl Manag Aquat Ecosyst 406: 01. [CrossRef] [EDP Sciences] [Google Scholar]
- Rimet F, Bouchez A, Tapolczai K. 2016. Spatial heterogeneity of littoral benthic diatoms in a large lake: monitoring implications. Hydrobiologia 771: 179–193. [Google Scholar]
- Sabater S, Gregory SV, Sedell JR. 1998. Community dynamics and metabolism of benthic algae colonizing wood and rock substrata in a forest stream. J Phycol 34: 561–567. [Google Scholar]
- Schaumburg J, Schranz C, Hofmann G, Stelzer D, Schneider S, Schmedtje U. 2004. Macrophytes and phytobenthos as indicators of ecological status in German lakes − a contribution to the implementation of the Water Framework Directive. Limnologica 34: 302–314. [CrossRef] [Google Scholar]
- Schaumburg J, Schranz C, Stelzer D, Hofmann G. 2007. Action instructions for the ecological evaluation of lakes for implementation of the EU Water Framework Directive: Macrophytes and Phytobenthos, Bavarian Environment Agency, Augsburg. [Google Scholar]
- Stenger-Kovács C, Buczko K, Hajnal E, Padisák J. 2007. Epiphytic, littoral diatoms as bioindicators of shallow lake trophic status: Trophic Diatom Index for Lakes (TDIL) developed in Hungary. Hydrobiologia 589: 141–154. [Google Scholar]
- Stevenson JR, Pan Y, Van Dam H. 2010. Assessing environmental conditions in rivers and streams with diatoms. In Smol JP, Stoermer EF, eds. The diatoms: applications for the environmental and earth sciences, Cambridge University Press, Cambridge, 11–40. [Google Scholar]
- Tan X, Zhang Q, Burford MA, Sheldon F, Bunn SE. 2017. Benthic diatom based indices for water quality assessment in two subtropical streams. Front Microbiol 8: 601. [Google Scholar]
- Taylor JC, de la Rey PA, van Rensburg L. 2005. Recommendations for the collection, preparation and enumeration of diatoms from riverine habitats for water quality monitoring in South Africa. Afr J Aquat Sci 30: 65–75. [Google Scholar]
- Trábert Z, Tihamér Kiss K, Várbíró G, Dobosy P, Grigorszky I, Ács É. 2017. Comparison of the utility of a frequently used diatom index (IPS) and the diatom ecological guilds in the ecological status assessment of large rivers. Fundam Appl Limnol/Archiv Für Hydrobiologie 189: 87–103. [Google Scholar]
- Trbojević I, Jovanović J, Kostić D, Popović S, Krizmanić J, Karadžić V, Simić GS. 2017. Structure and succession of periphyton in an urban reservoir: artificial substrate specificity. Oceanol Hydrobiol Stud 46: 379. [Google Scholar]
- Trbojević I. 2018. Analysis of periphyton developed on artificial substrates in the Sava Lake and the Vrutci Lake. Doctoral dissertation, University of Belgrade, Belgrade, Serbia. Available at: https://nardus.mpn.gov.rs/handle/123456789/10710?locale-attribute=sr_RS (In Serbian). [Google Scholar]
- Trbojević IS, Predojević DD, Subakov-Simić GV, Krizmanić JŽ. 2019. Periphytic diatoms in the presence of a cyanobacterial bloom: A case study of the Vrutci Reservoir in Serbia. Arch Biol Sci 71: 215–223. [Google Scholar]
- Zhang N, Li H, Jeppesen E, Li W. 2013. Influence of substrate type on periphyton biomass and nutrient state at contrasting high nutrient levels in a subtropical shallow lake. Hydrobiologia 710: 129–141. [CrossRef] [Google Scholar]
Cite this article as: Trbojević IS, Popović SS, Milovanović VV, Predojević DD, Subakov Simić GV, Jakovljević OS, Krizmanić JŽ. 2021. Substrate type selection in diatom based lake water quality assessment. Knowl. Manag. Aquat. Ecosyst., 422, 21.
All Tables
Colored sheme of water quality classification based on IBD index values (according to Prygiel and Coste, 2000).
Qualitative inventory − complete list of diatoms detected, when both artificial and natural substrates are considered. For taxa that occurred in quantitative analyses, codes (from Omnidia) are listed.
Codes labeled with * are for the taxa used for IBD calculation.
All Figures
 |
Fig. 1 Map showing sampling sites for artificial substrates (glass, ceramic, willow and yew tree tiles) and (b) natural substrate (pebble stone) in Sava Lake, Serbia. |
| In the text | |
 |
Fig. 2 Dynamics of (a) diversity and (b) equitability of the diatom community during the study period in Sava Lake. |
| In the text | |
 |
Fig. 3 The relationship between diatom taxa and (a) environmental parameters and (b) substrates. |
| In the text | |
 |
Fig. 4 Diatom indices characterized by number values (0–20), and also by water quality classes they indicate according Prygiel and Coste's (2000) categorization. Empty fields are for the lost/damaged samples. |
| In the text | |
 |
Fig. 5 The relationship between diatom indices and (a) environmental parameters and (b) substrates. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


